Study Notes
Overview
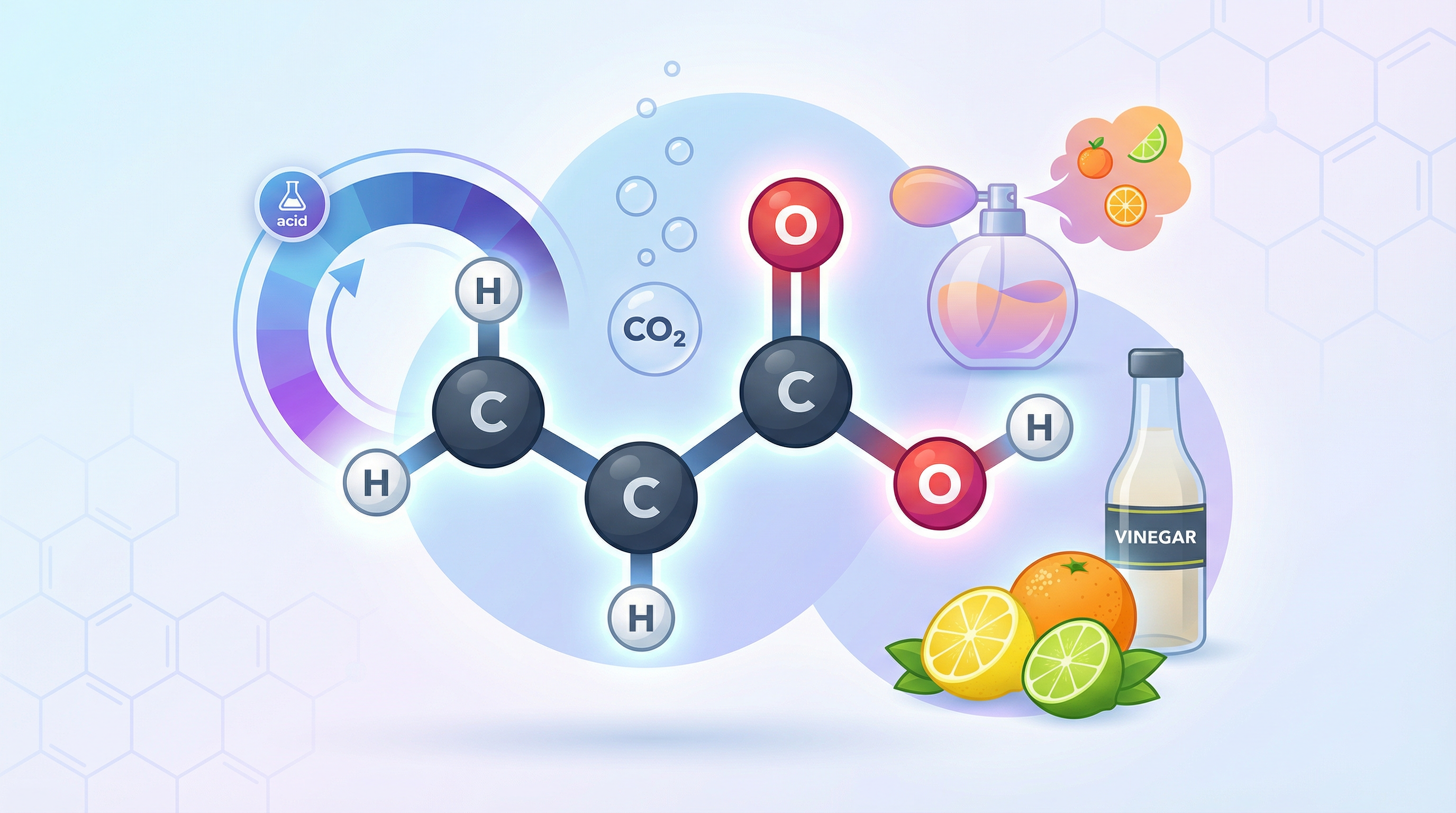
Carboxylic acids are a homologous series of organic compounds that are defined by the presence of the carboxyl functional group (-COOH). This topic is a cornerstone of the AQA GCSE Chemistry specification, and a solid understanding is crucial for success in the exam. Carboxylic acids are not just an abstract concept; they are present in many everyday substances, from the vinegar in your kitchen (ethanoic acid) to the stings of ants (methanoic acid). In the exam, you will be expected to recall the names and structures of the first four carboxylic acids, describe their reactions, and for higher tier students, explain the difference between weak and strong acids. This guide will walk you through all the key concepts, providing you with the knowledge and exam technique to secure top marks.
Key Concepts
Concept 1: The Carboxyl Functional Group
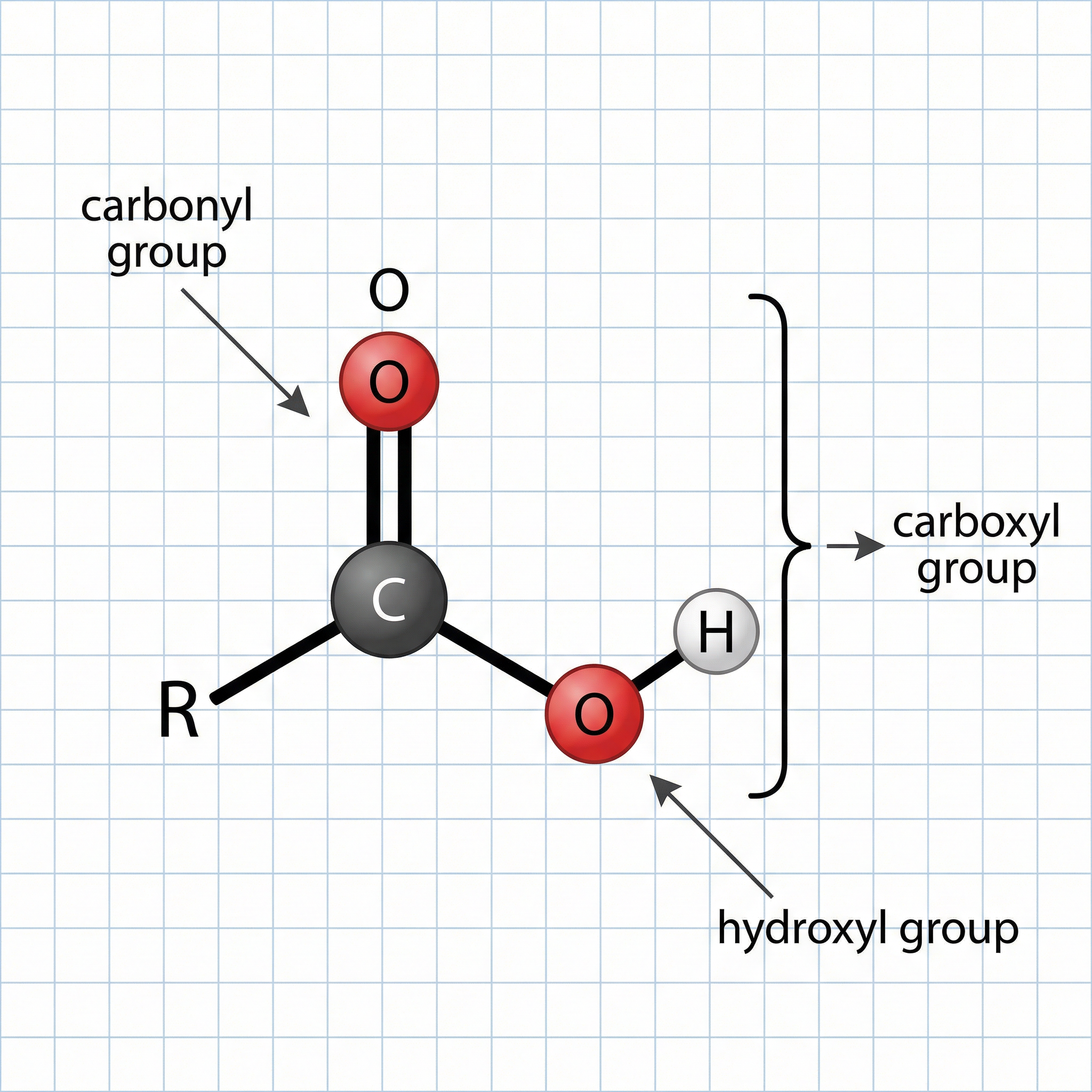
The properties and reactions of carboxylic acids are determined by the carboxyl functional group, -COOH. This group consists of a carbonyl group (C=O) and a hydroxyl group (-OH) attached to the same carbon atom. It is essential that you can draw the displayed formula of this functional group, showing all the bonds. A common mistake is to write -OH without showing the single bond between the oxygen and hydrogen atoms, which will result in losing a mark in the exam.
Concept 2: The Homologous Series
Carboxylic acids form a homologous series, which means they have the same functional group and similar chemical properties. You need to know the names and structures of the first four members of this series:
- Methanoic acid: HCOOH
- Ethanoic acid: CH3COOH
- Propanoic acid: CH3CH2COOH
- Butanoic acid: CH3CH2CH2COOH
Notice the systematic naming: the prefix indicates the number of carbon atoms (meth- for 1, eth- for 2, prop- for 3, and but- for 4), and the suffix “-oic acid” indicates that it is a carboxylic acid.
Concept 3: Reactions of Carboxylic Acids
There are three main reactions of carboxylic acids that you need to know for your exam:
-
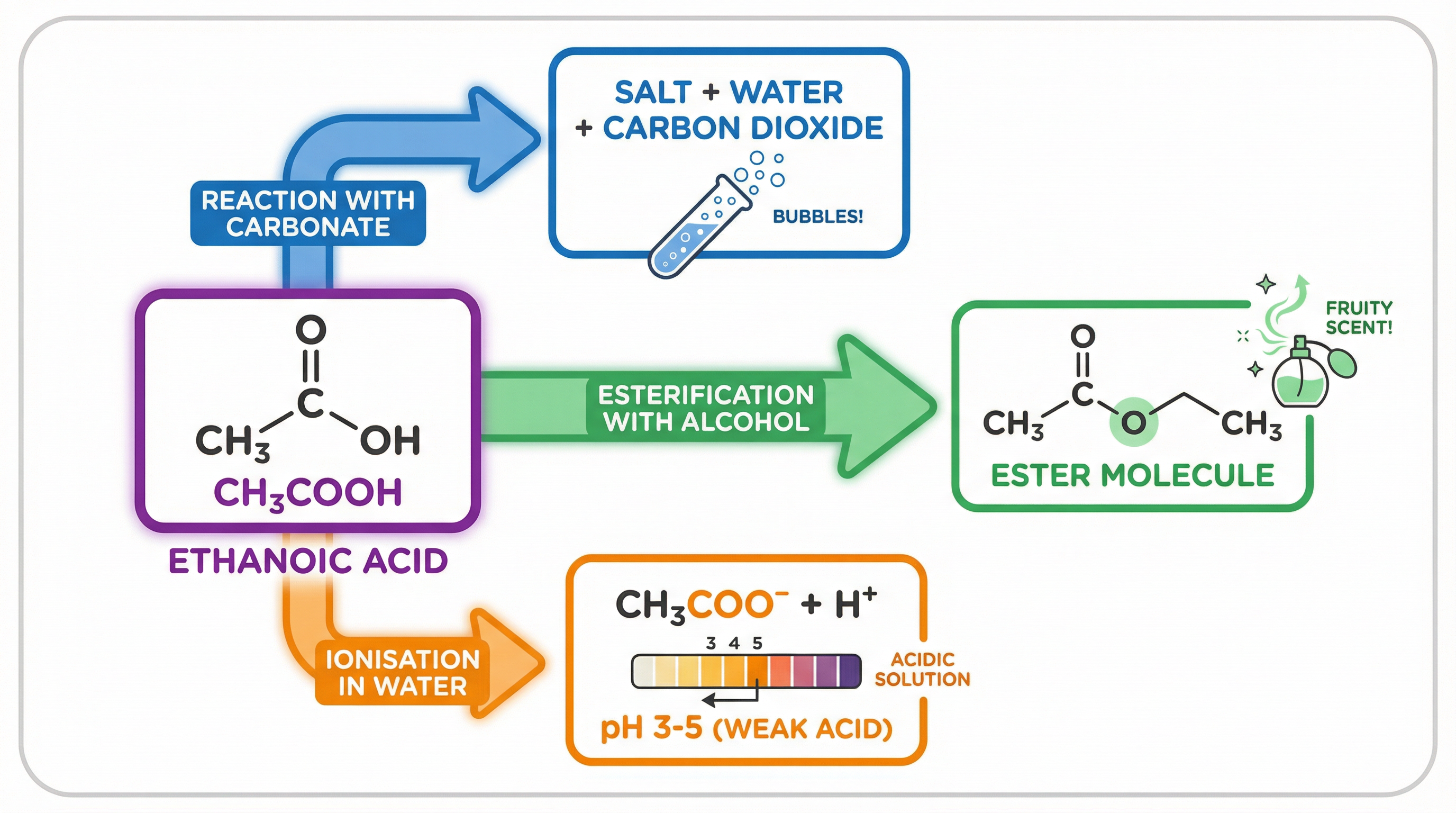
Reaction with carbonates: Carboxylic acids react with carbonates to produce a salt, water, and carbon dioxide. This is a standard test for acids, and the production of carbon dioxide is observed as effervescence (fizzing). For example, the reaction of ethanoic acid with sodium carbonate produces sodium ethanoate, water, and carbon dioxide.
-
Ionisation in water: Carboxylic acids are weak acids. This means that when they are dissolved in water, they only partially ionise, releasing a small proportion of their hydrogen atoms as H+ ions. This is a key concept for higher tier students, who need to be able to distinguish between acid strength (the degree of ionisation) and concentration (the amount of acid dissolved in a given volume of water).
-
Esterification: Carboxylic acids react with alcohols in the presence of an acid catalyst (usually concentrated sulfuric acid) to form esters and water. Esters are known for their sweet, fruity smells and are used in perfumes and food flavourings. For example, ethanoic acid reacts with ethanol to produce ethyl ethanoate and water.
Mathematical/Scientific Relationships
There are no complex mathematical formulas to learn for this topic at GCSE level. However, you will need to be able to write and balance chemical equations for the reactions of carboxylic acids. For example:
- Reaction with carbonate: 2CH3COOH(aq) + Na2CO3(s) -> 2CH3COONa(aq) + H2O(l) + CO2(g)
- Esterification: CH3COOH(l) + CH3CH2OH(l) <=> CH3COOCH2CH3(l) + H2O(l)
Practical Applications
Carboxylic acids have many real-world applications. Ethanoic acid is the main component of vinegar, which is used in cooking and as a cleaning agent. Citric acid, a more complex carboxylic acid, is found in citrus fruits and is used as a flavouring and preservative in the food and drink industry. Carboxylic acids are also used in the production of soaps, detergents, and polymers.
