Study Notes
Overview
Welcome to the study of Alcohols, a fundamental topic in organic chemistry that bridges concepts from bonding and structure to energy changes and industrial processes. For your Edexcel GCSE, this topic (2.12) focuses on the properties and reactions of the alcohol homologous series, particularly ethanol. Understanding alcohols is crucial as it not only explains the behaviour of everyday substances like fuels and solvents but also serves as a foundation for more advanced organic chemistry. Examiners frequently test this area through structured questions, comparisons, and calculations, often assessing your ability to link molecular structure to physical and chemical properties. This guide will equip you with the core knowledge and exam technique needed to excel.
Key Concepts
Concept 1: The Alcohol Homologous Series
Alcohols form a homologous series, which is a family of organic compounds with the same functional group and similar chemical properties. The functional group for alcohols is the hydroxyl group, -OH. It is this group that dictates how alcohols react.
For exam purposes, you must be able to draw the first four members of the series: Methanol (CH3OH), Ethanol (C2H5OH), Propanol (C3H7OH), and Butanol (C4H9OH). A critical point that candidates often miss is in drawing displayed formulas: the hydroxyl group must be shown as -O-H, with a clear bond between the oxygen and hydrogen. Simply writing ‘-OH’ will not be awarded the mark.
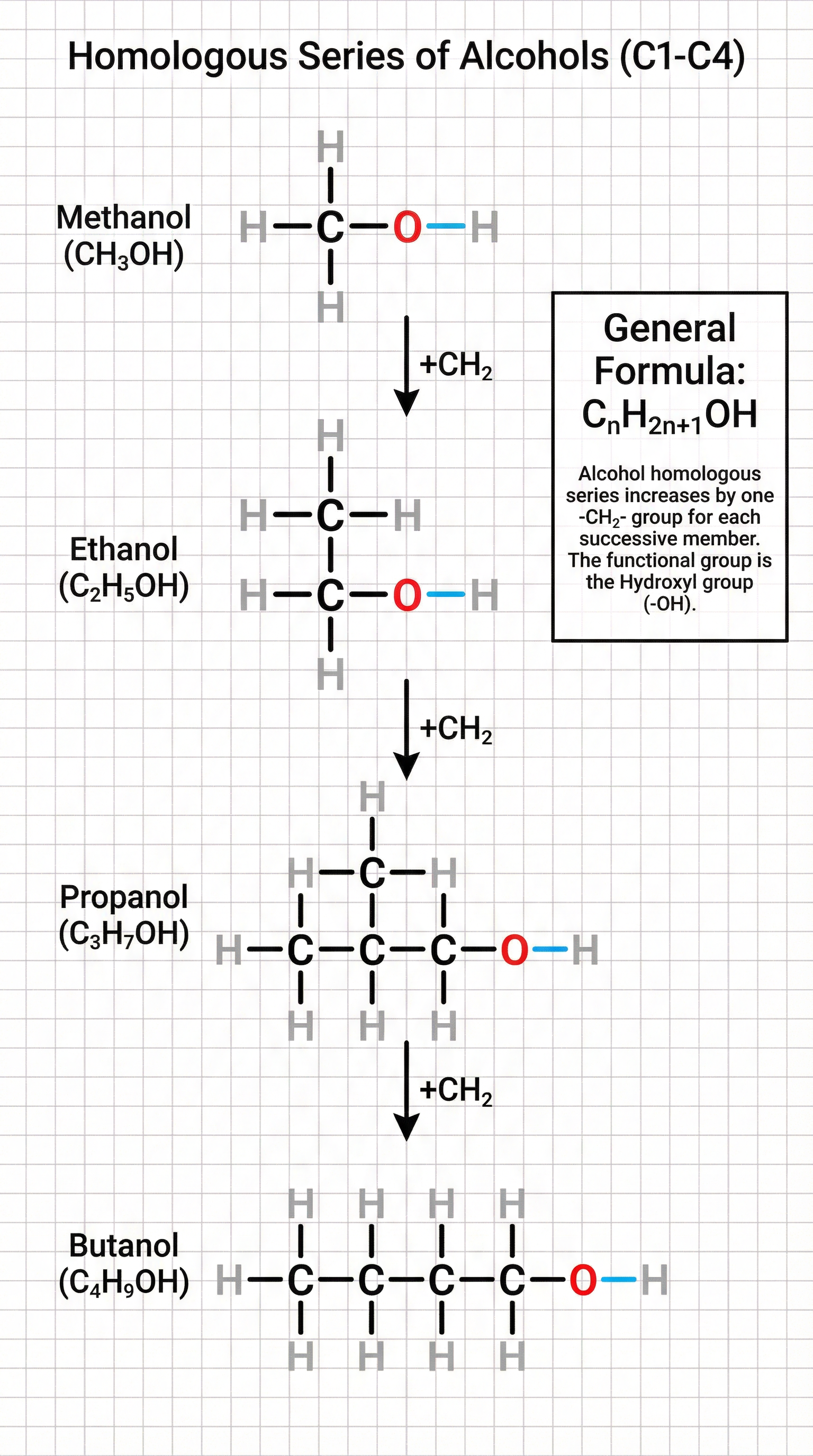
The general formula for the alcohol homologous series is CnH2n+1OH. As the carbon chain length increases, the physical properties change predictably. For instance, the boiling points increase due to stronger intermolecular forces between the larger molecules.
Concept 2: Structural Isomerism in Alcohols
As the carbon chain grows, the position of the -OH group can change, leading to structural isomers. These are molecules with the same molecular formula but a different structural arrangement of atoms. For your GCSE, you need to understand the isomers of propanol (C3H8O).
- Propan-1-ol: The -OH group is on a terminal (end) carbon atom.
- Propan-2-ol: The -OH group is on the central carbon atom.
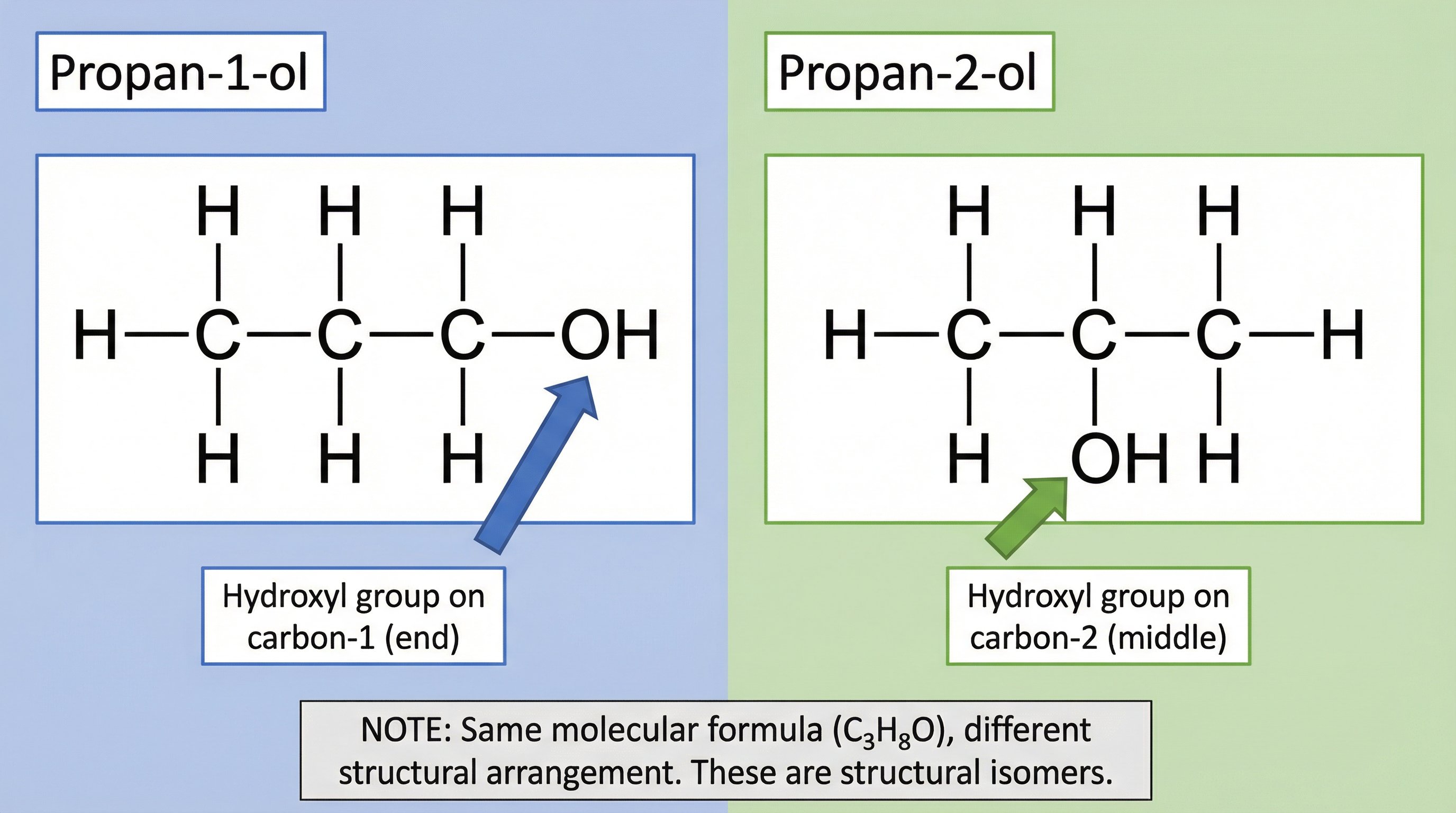
This difference in structure, while subtle, can affect the chemical properties of the isomers, a concept explored more at A-Level. If an exam question asks you to draw ‘propanol’ without specifying, Propan-1-ol is the standard expected answer.
Concept 3: Production of Ethanol
Ethanol is a significant industrial chemical, and you need to know two methods of production. This is a classic 6-mark comparison question, so structuring your answer is key.
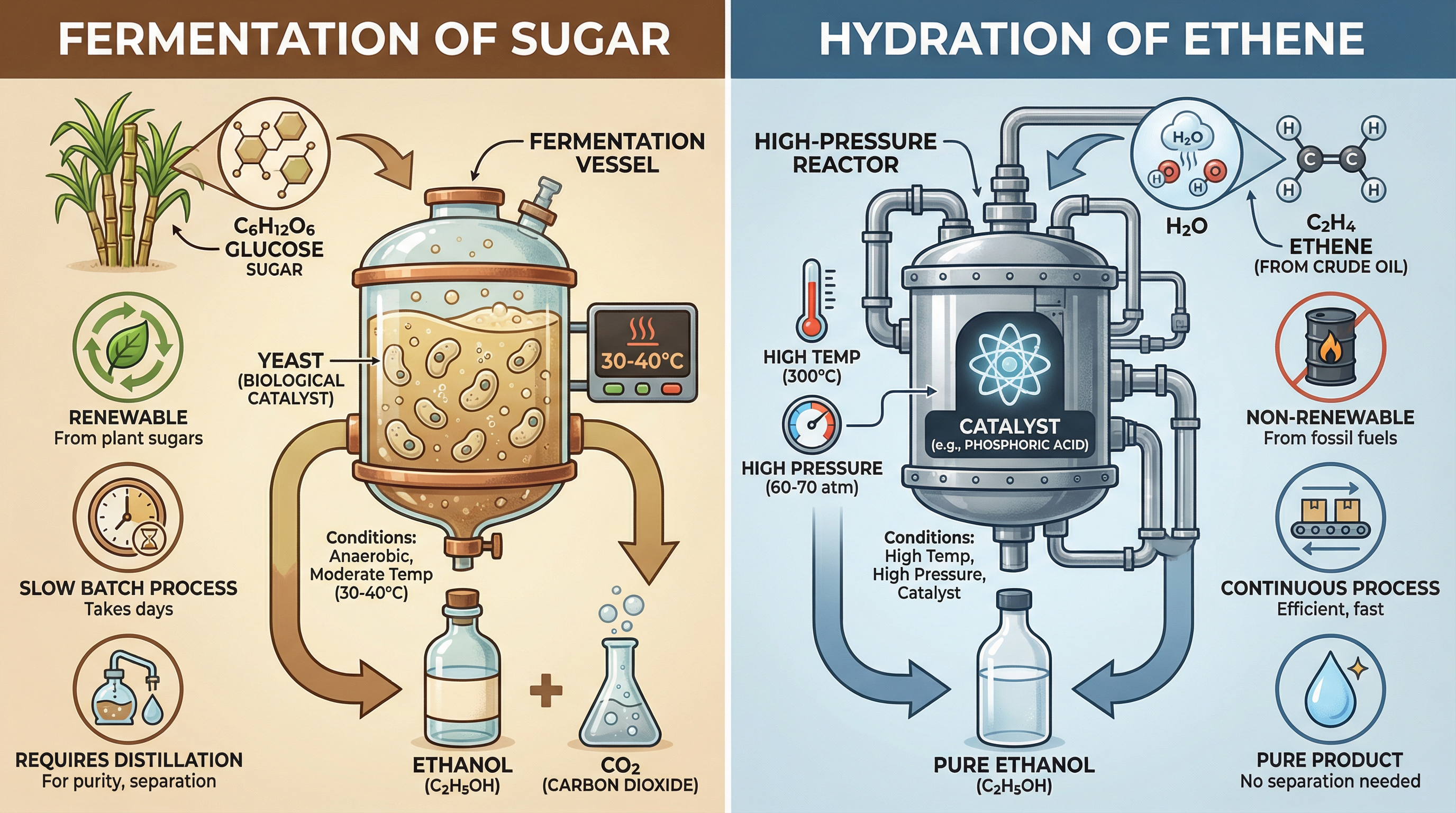
Method 1: FermentationThis is a biological process using a renewable resource.
- Raw Material: Sugars (e.g., glucose from sugar cane or corn).
- Process: Yeast is added to a sugar solution. The yeast contains enzymes that catalyse the breakdown of glucose into ethanol and carbon dioxide.
- Conditions: Anaerobic (no oxygen) and a warm temperature (optimally 30-40°C). If oxygen is present, ethanoic acid is produced instead. If it is too hot, the enzymes in the yeast will denature.
- Product: An aqueous solution of ethanol is produced, which must be purified by fractional distillation. This is a batch process, meaning it is relatively slow.
- Equation: C6H12O6(aq) → 2C2H5OH(aq) + 2CO2(g)
Method 2: Hydration of EtheneThis is a continuous industrial process using a non-renewable resource.
- Raw Material: Ethene (C2H4), obtained from the cracking of crude oil fractions.
- Process: Ethene gas is reacted with steam.
- Conditions: High temperature (300°C) and high pressure (60-70 atm), with a phosphoric acid catalyst.
- Product: Pure ethanol is produced. This is a fast, continuous process.
- Equation: C2H4(g) + H2O(g) ⇌ C2H5OH(g)
Concept 4: Reactions of Alcohols
1. Complete CombustionAlcohols are flammable and can be used as fuels. They undergo complete combustion in a plentiful supply of oxygen to produce carbon dioxide and water. A common mistake is forgetting to account for the oxygen atom already present in the alcohol molecule when balancing the equation.
Example (Ethanol): C2H5OH(l) + 3O2(g) → 2CO2(g) + 3H2O(l)
Examiner Tip: To balance, start with Carbon, then Hydrogen, and do Oxygen last. Count the oxygens on the product side, subtract the one oxygen from the alcohol molecule, and then determine the required number of O2 molecules.
**2. Oxidation (Higher Tier Only)**Alcohols can be oxidised by an oxidising agent (such as acidified potassium dichromate(VI)) to produce carboxylic acids. For example, the oxidation of ethanol produces ethanoic acid. This reaction is responsible for wine turning into vinegar if left exposed to air.
Example (Ethanol to Ethanoic Acid): C2H5OH + [O] → CH3COOH + H2O
Note: [O] represents oxygen from the oxidising agent. During this reaction, the orange colour of the dichromate(VI) ions turns green.
Mathematical/Scientific Relationships
- General Formula: CnH2n+1OH. This is a formula you must memorise. It allows you to predict the molecular formula of any alcohol in the series.
- Balancing Combustion Equations: This is a key mathematical skill. Always follow the C → H → O order for balancing. Remember to subtract the oxygen atom in the alcohol molecule itself when balancing the oxygen atoms.
Practical Applications
- Fuels: Ethanol is added to petrol (bioethanol) to reduce reliance on fossil fuels.
- Solvents: Alcohols are excellent solvents and are used in perfumes, aftershaves, and inks because they can dissolve substances that water cannot, and they evaporate easily.
- Antiseptics: Ethanol and propanol are used in hand sanitisers and antiseptic wipes to kill microorganisms.
- Alcoholic Drinks: Ethanol, produced by fermentation, is the alcohol found in beer, wine, and spirits.
