Study Notes
Overview
Alkanes represent the foundational homologous series in organic chemistry, a group of saturated hydrocarbons that are fundamental to understanding fuels and the structure of carbon compounds. For Edexcel GCSE Chemistry candidates, mastering alkanes is not just about memorising a few facts; it is about building a core understanding of chemical principles that will be revisited in more complex topics. This topic, specification point 2.10, requires a solid grasp of the general formula CnH2n+2, the ability to draw and interpret displayed formulae, and a clear understanding of how physical properties like boiling point and viscosity change with molecular size. Furthermore, a key assessment area is combustion, where candidates must be able to construct and balance symbol equations for both complete and incomplete reactions. Exam questions often combine these elements, asking students to apply their knowledge to unfamiliar alkanes or to explain trends using precise scientific language. A strong performance in this area demonstrates a candidate's ability to link structure to properties and to handle quantitative chemical equations, skills that are highly valued by examiners.
Key Concepts
Concept 1: Homologous Series and General Formula
Alkanes are the simplest homologous series of hydrocarbons. A homologous series is a family of compounds with the same general formula and similar chemical properties. The 'hydrocarbon' part of the name tells you they are made up of hydrogen (H) and carbon (C) atoms only. The term that examiners expect you to use and understand is saturated. A saturated hydrocarbon contains only single covalent bonds between its carbon atoms. This is the key feature that distinguishes alkanes from other homologous series like alkenes, which are unsaturated.
Every homologous series has a general formula, which is a powerful tool for working out the molecular formula of any member of the series. For alkanes, this is a non-negotiable fact to memorise:
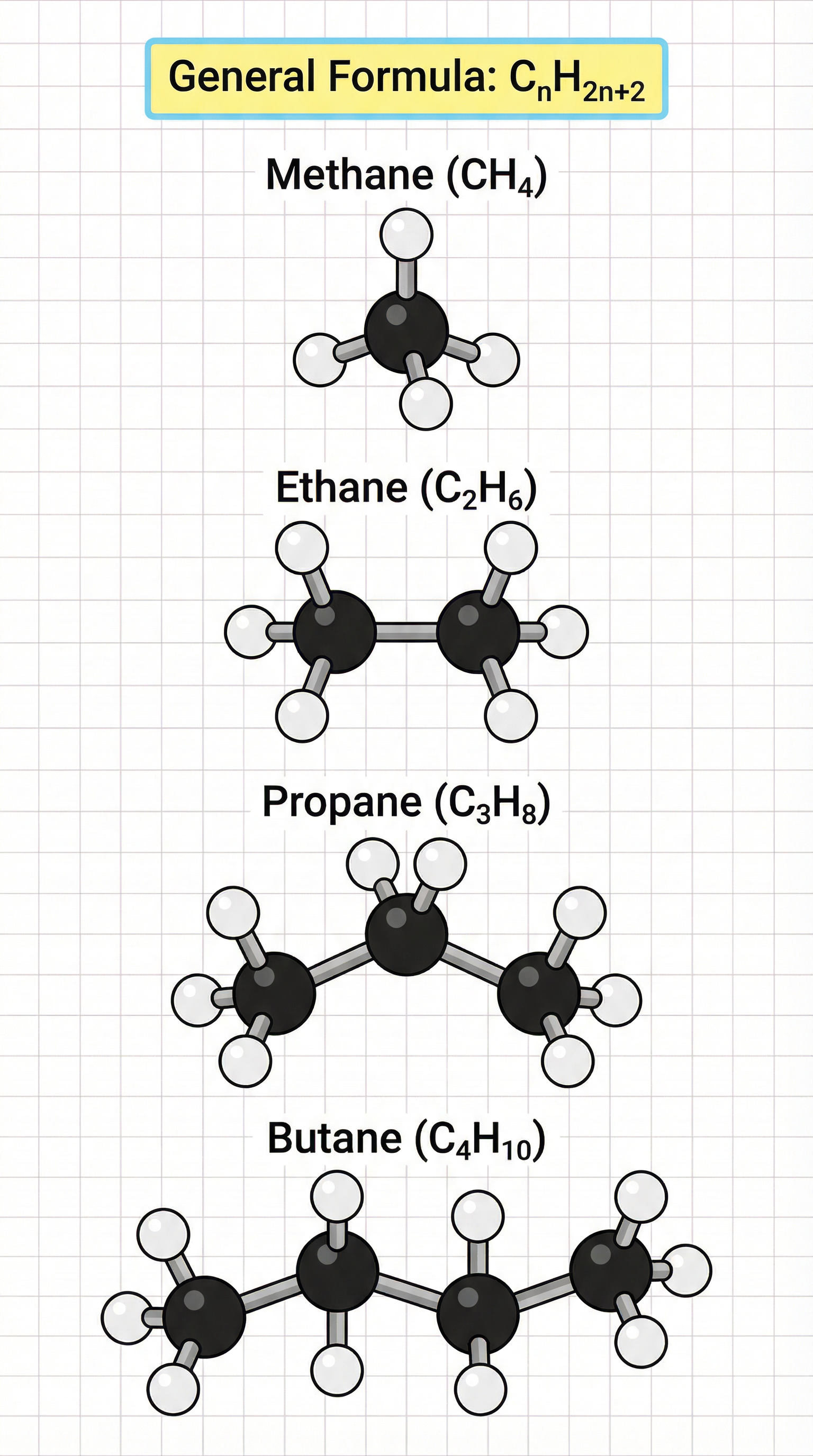
General Formula: CnH2n+2Here, 'n' represents the number of carbon atoms. To find the number of hydrogen atoms, you simply double the number of carbon atoms and add two. For example, if an alkane has 5 carbon atoms (n=5), the number of hydrogen atoms will be (2 * 5) + 2 = 12. Therefore, the molecular formula for pentane is C5H12. This formula is frequently tested, often as a 1-mark recall question or as the first step in a longer problem.
Concept 2: Structure and Displayed Formulae
To earn marks on structure questions, candidates must be able to draw displayed formulae. A displayed formula shows all the atoms and all the covalent bonds within a molecule. It is a 2D representation of a 3D structure. The rules are simple but strict:
- Carbon atoms form 4 bonds.
- **Hydrogen atoms form 1 bond.**Examiners are meticulous when marking these. A missing bond, a carbon with 5 bonds, or a hydrogen with 2 bonds will result in lost marks. It is crucial to be neat and clear. The first four alkanes, which you must be able to name and draw, are:
- Methane (CH4)
- Ethane (C2H6)
- Propane (C3H8)
- Butane (C4H10)
Concept 3: Trends in Physical Properties
As the carbon chain in an alkane molecule gets longer, its physical properties change in a predictable way. The two key trends you need to know for your exam are:
- Boiling Point: The boiling point of alkanes increases as the chain length increases.
- Viscosity: The viscosity of alkanes increases as the chain length increases. Viscosity is a measure of a fluid's resistance to flow – essentially, how 'thick' it is. Longer alkanes are more viscous.
To gain full credit for explaining these trends, you must use the correct scientific terminology. The explanation hinges on intermolecular forces. These are the weak forces of attraction between molecules. It is a common and costly mistake to talk about the breaking of strong covalent bonds within the molecules.
Explanation: Longer carbon chains have a larger surface area. This leads to stronger intermolecular forces of attraction between the molecules. More energy is required to overcome these stronger forces, resulting in a higher boiling point. For viscosity, the longer chains are more likely to get tangled up with each other, making it more difficult for the liquid to flow.
Concept 4: Combustion Reactions
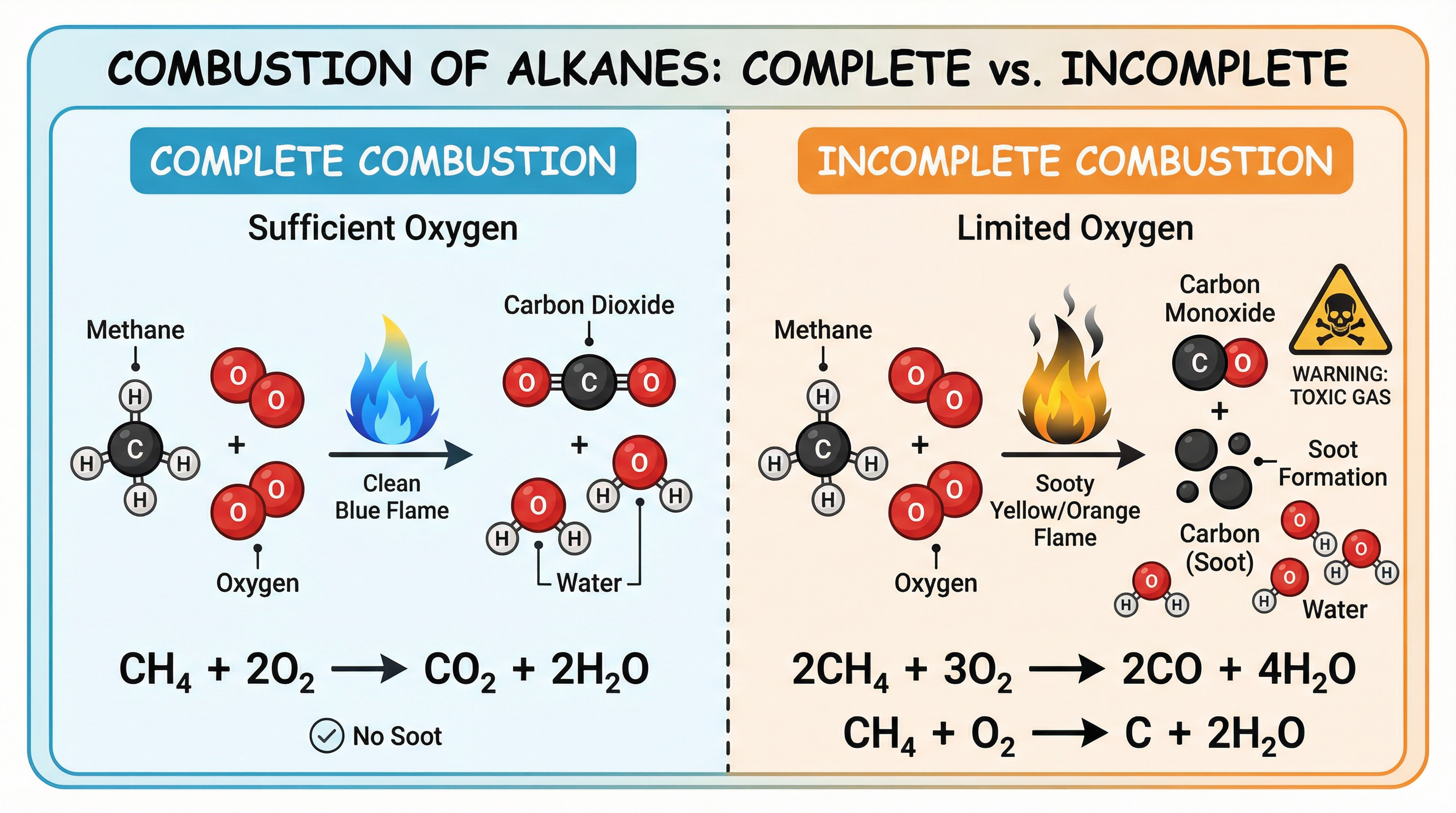
Alkanes are excellent fuels, meaning they release a large amount of energy when they burn. This process is called combustion, which is a rapid reaction with oxygen. There are two types of combustion that you must be able to describe and for which you must write balanced symbol equations.
-
Complete Combustion: This occurs when there is a plentiful supply of oxygen. The alkane burns cleanly with a blue flame to produce only two products: carbon dioxide (CO2) and water (H2O).
Word Equation: Alkane + Oxygen → Carbon Dioxide + Water
Example (Methane): CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) -
Incomplete Combustion: This occurs when the supply of oxygen is limited. It is a less efficient reaction that produces a sooty, yellow-orange flame. The products can include carbon monoxide (CO), a toxic gas, and/or carbon (C), which is seen as soot, in addition to water.
Word Equation (producing CO): Alkane + Oxygen → Carbon Monoxide + Water
Example (Methane): 2CH4(g) + 3O2(g) → 2CO(g) + 4H2O(l)Word Equation (producing C): Alkane + Oxygen → Carbon + Water
Example (Methane): CH4(g) + O2(g) → C(s) + 2H2O(l)
Examiners will specify which type of combustion to write an equation for. Pay close attention to the command words and the context of the question.
Mathematical/Scientific Relationships
General Formula for Alkanes
This is the most important mathematical relationship for this topic. It is a rule that defines the composition of any alkane.
- Formula:
CnH2n+2 - n: The number of carbon atoms in the molecule.
- 2n+2: The number of hydrogen atoms in the molecule.
- When to use: Use this to determine the molecular formula of an alkane when given the number of carbon atoms, or to identify a compound as an alkane from its formula.
- Status: Must memorise. This is not given on the formula sheet.
Balancing Combustion Equations
Balancing equations is a key mathematical skill in chemistry. For combustion of alkanes, follow a set order:
- Balance Carbon (C) atoms first. The number of CO2 or CO molecules on the right will match the number of C atoms in the alkane on the left.
- Balance Hydrogen (H) atoms second. The number of H2O molecules on the right will be half the number of H atoms in the alkane.
- Balance Oxygen (O) atoms last. Count the total number of oxygen atoms on the right-hand side (in CO2/CO and H2O) and then place the appropriate coefficient in front of O2 on the left. If you get an odd number of oxygen atoms on the right, you may need to double all the other coefficients.
Practical Applications
Alkanes are not just an abstract topic; they are central to modern life. The main source of alkanes is crude oil, a fossil fuel. Crude oil is a complex mixture of hydrocarbons, which are separated into useful products by a process called fractional distillation. This process separates the hydrocarbons based on their chain length and boiling points. Shorter-chain alkanes with lower boiling points rise higher up the fractionating column, while longer-chain alkanes with higher boiling points remain at the bottom.
- Gases (e.g., Methane, Propane): Used for domestic heating and cooking.
- Petrol (Gasoline): A mixture of alkanes (typically C5-C12) used as fuel for cars.
- Kerosene: Used as jet fuel and for paraffin lamps.
- Diesel: Used as fuel for diesel engines.
- Fuel Oil and Bitumen: Very long-chain alkanes used for fuel for ships, lubricating oils, and for surfacing roads.
Understanding the properties of alkanes explains their uses. For example, petrol must be volatile (evaporate easily) to work in an engine, which is why it is made of shorter-chain alkanes with low boiling points.
