Study Notes
Overview
Balancing chemical equations is a fundamental skill in chemistry, and for Edexcel GCSE, it falls under specification point 1.10. It's the process of ensuring that a chemical equation adheres to the Law of Conservation of Mass, which states that no atoms are created or destroyed during a chemical reaction. This means you must have the exact same number of atoms of each element on the reactants side (left) as you do on the products side (right). Examiners frequently test this, both in simple balancing tasks and as the first step in more complex calculation questions (stoichiometry). Mastering this not only secures direct marks but is essential for understanding topics like reacting masses, titrations, and atom economy. A typical exam question might ask you to simply balance a given equation, or for higher marks, to write a balanced equation from scratch, including state symbols.
Key Concepts
Concept 1: The Law of Conservation of Mass
This is the bedrock principle. Imagine you're building with LEGO bricks. You start with a pile of red, blue, and yellow bricks. After you've built your model, you still have the same number of red, blue, and yellow bricks, they're just arranged differently. A chemical reaction is the same. The atoms are just rearranged to form new substances. No atoms appear out of nowhere, and none vanish. Credit is given in exams for demonstrating this understanding by ensuring atom counts are equal on both sides of the equation.
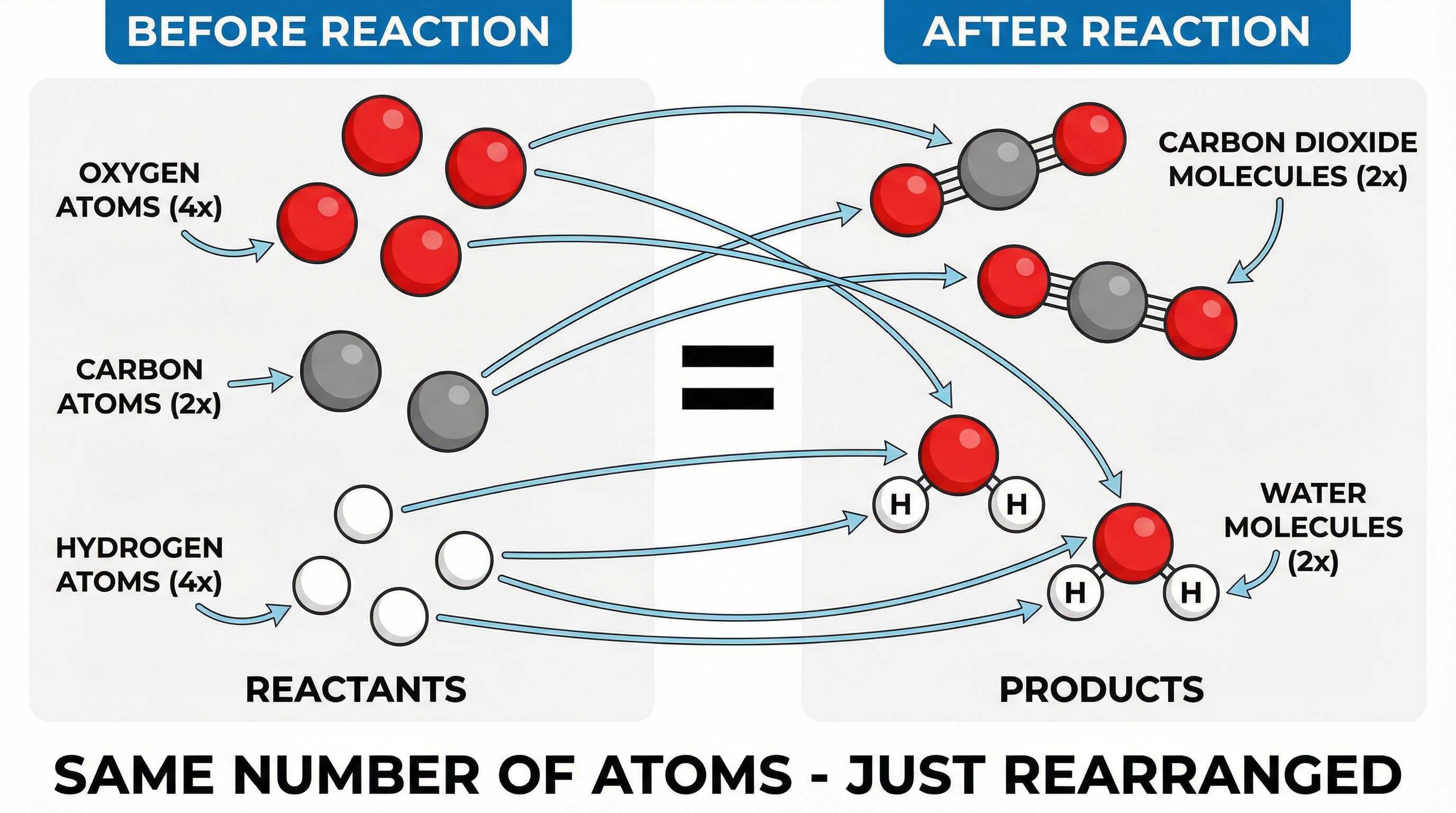
Example: In the reaction between methane and oxygen (CH₄ + O₂ → CO₂ + H₂O), you start with Carbon, Hydrogen, and Oxygen atoms, and you end with the same atoms, just bonded into different molecules.
Concept 2: Reactants, Products, and State Symbols
- Reactants: The substances you start with. They are always written on the left-hand side of the equation.
- Products: The new substances formed in the reaction. They are written on the right-hand side.
- State Symbols: These tell you the physical state of each substance. They are often required for full marks. The four state symbols are:
- (s) - solid
- (l) - liquid
- (g) - gas
- (aq) - aqueous (dissolved in water)
Concept 3: Coefficients vs. Subscripts
This is the most common area for mistakes.
- Subscripts: These are the small numbers written after an atom in a formula (e.g., the '2' in H₂O). You must never change the subscripts. Changing them alters the chemical identity of the substance (e.g., changing H₂O to H₂O₂ changes water to hydrogen peroxide).
- Coefficients: These are the large numbers placed in front of a chemical formula (e.g., the '2' in 2H₂O). These are the only numbers you can change. A coefficient multiplies every atom in the formula that follows it. So, 2H₂O means you have two water molecules, giving you 4 Hydrogen atoms and 2 Oxygen atoms in total.
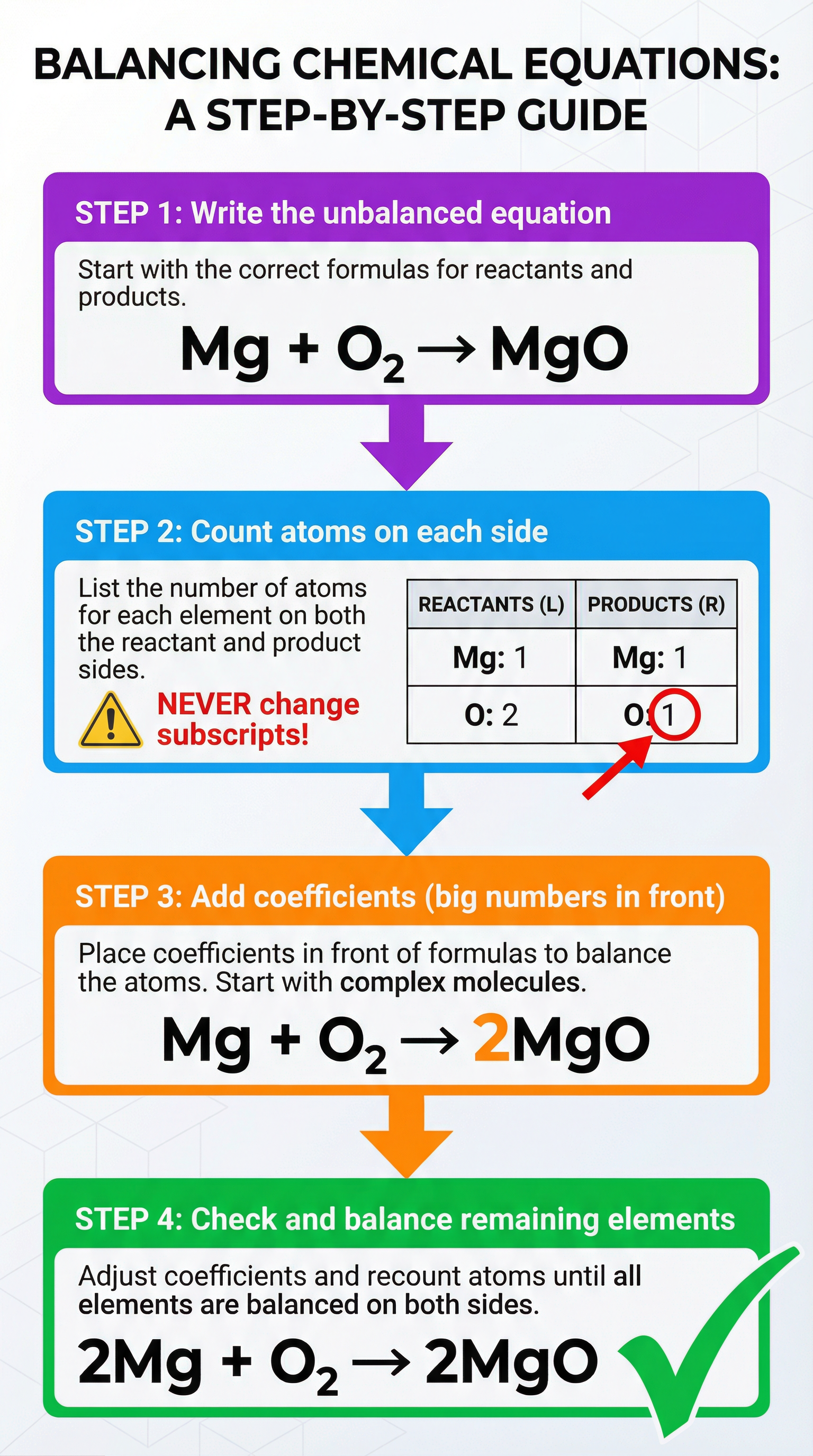
Mathematical/Scientific Relationships
The core relationship is simple:
Total number of atoms of Element X in Reactants = Total number of atoms of Element X in ProductsTo balance an equation, you follow a systematic process:
- Write the unbalanced equation: Ensure all chemical formulae are correct.
- Count the atoms: Tally the number of atoms of each element on both the reactant and product sides.
- Add coefficients: Place coefficients in front of the chemical formulae to multiply the atoms until they are equal on both sides.
- Check your work: Do a final count to ensure all elements are balanced and that the coefficients are in their simplest whole-number ratio.
Practical Applications
Balancing equations is vital in the real world. For example, in the Haber process for making ammonia (N₂ + 3H₂ → 2NH₃), chemists need to know the exact ratio of nitrogen to hydrogen to get the maximum yield of ammonia fertilizer. In pharmaceuticals, precise reactions are needed to synthesize drugs safely and efficiently. Even in the kitchen, the reaction that makes cakes rise (sodium bicarbonate reacting with an acid) involves a balanced chemical equation! This topic is also linked to the required practical involving measuring energy changes (calorimetry), where knowing the balanced equation is the first step to calculating the molar enthalpy change.
