Study Notes
Overview
Drawing dot and cross diagrams is a fundamental skill in chemistry, allowing us to visualise the invisible world of electrons and chemical bonds. For your Edexcel GCSE exam, this isn't just about drawing circles; it's about demonstrating a precise understanding of how and why atoms bond. This topic, specification point 3.10, is crucial because it forms the basis of understanding structure, properties, and reactions. Examiners frequently use it to test your grasp of atomic structure (Topic 1) and how it dictates the type of bonding that occurs. A typical exam question will ask you to 'Draw a dot and cross diagram to show the bonding in...' for a specific ionic or covalent substance, often with the constraint 'show outer electrons only'. Mastering this will give you a powerful tool to deconstruct and explain the properties of matter.
Key Concepts
Concept 1: Ionic Bonding - The Great Electron Transfer
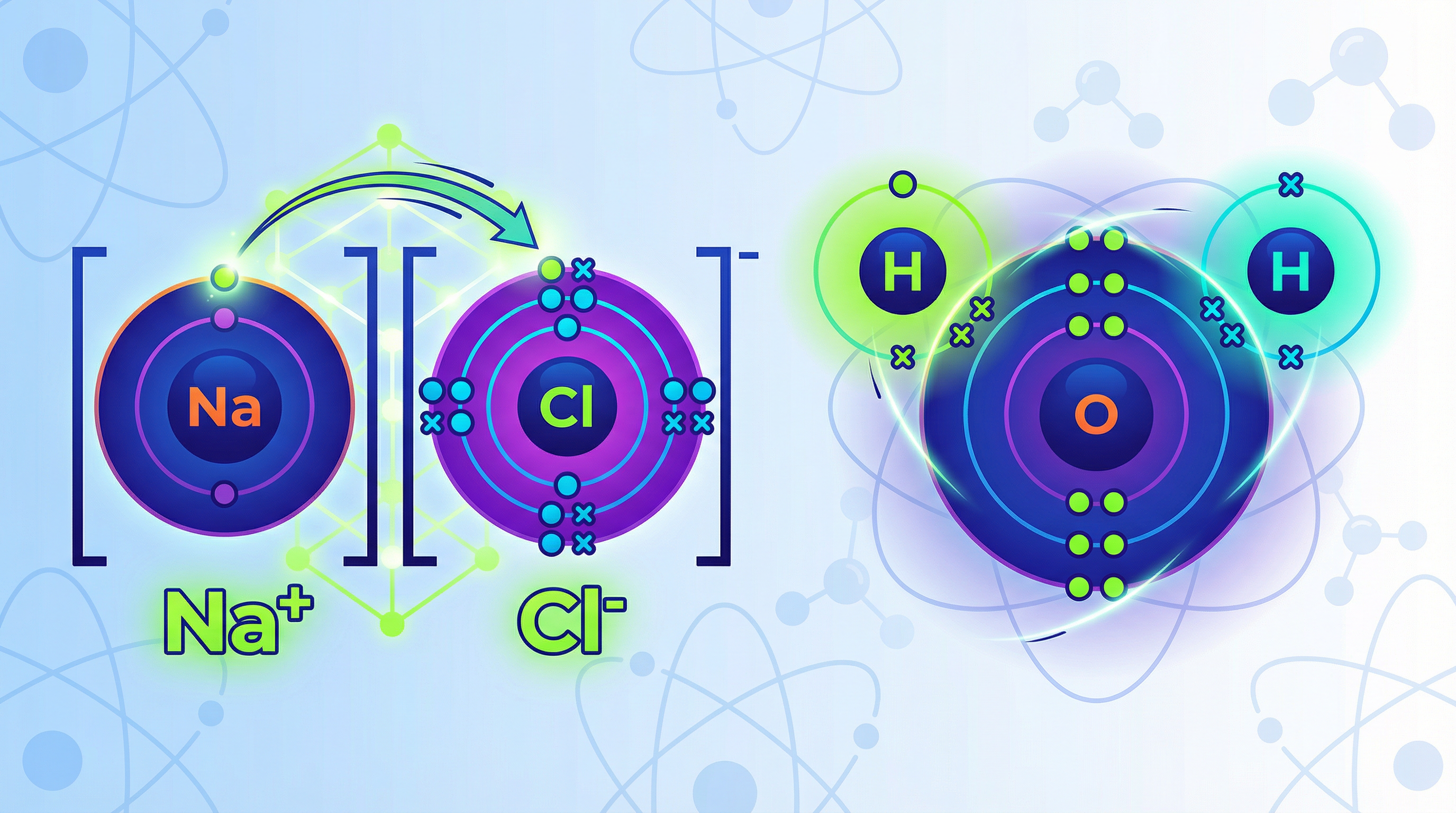
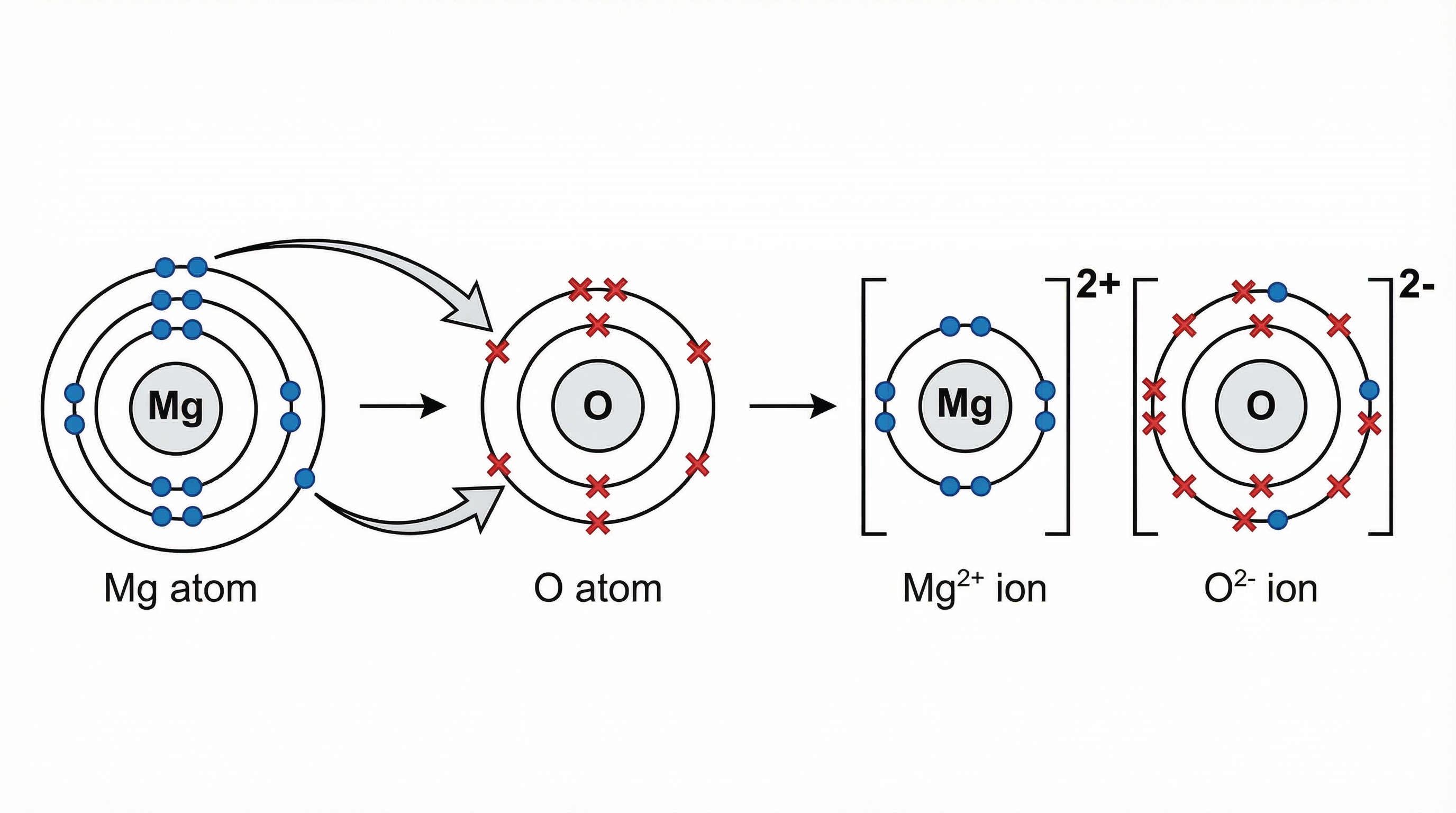
Ionic bonding occurs between a metal and a non-metal. The key principle here is the transfer of electrons. Metal atoms, found on the left side of the periodic table, have few electrons in their outer shell. They can achieve a stable, full outer shell (like a noble gas) most easily by losing these electrons. When they lose negatively charged electrons, they form positive ions (cations).
Non-metal atoms, on the right of the periodic table, have nearly full outer shells. They achieve stability by gaining electrons to complete their outer shell, forming negative ions (anions).
The result is a strong electrostatic force of attraction between the oppositely charged ions. This is the ionic bond.
Example: Consider Sodium (Na) and Chlorine (Cl). Sodium is in Group 1, with one outer electron. Chlorine is in Group 7, with seven. Sodium transfers its electron to chlorine. Sodium becomes Na⁺ and chlorine becomes Cl⁻. The final diagram shows two separate ions in square brackets with their charges displayed. Crucially, their electron shells do not overlap.
Concept 2: Covalent Bonding - The Art of Sharing
Covalent bonding occurs between non-metal atoms only. These atoms are in a similar position – they all need to gain electrons to achieve a stable outer shell, but none are willing to give them up. The solution is to share electrons. A covalent bond is a shared pair of electrons.
When drawing these, the outer electron shells of the atoms overlap. The shared electrons are placed in this overlapping section. Each shared pair constitutes one covalent bond.
Example: In a water molecule (H₂O), oxygen (Group 6) needs two electrons, and each hydrogen (Group 1) needs one. The oxygen atom shares one electron with each of the two hydrogen atoms. The circles overlap, and in each overlap zone, there is one electron from oxygen (e.g., a cross) and one from hydrogen (e.g., a dot). This allows all three atoms to have a full outer shell.
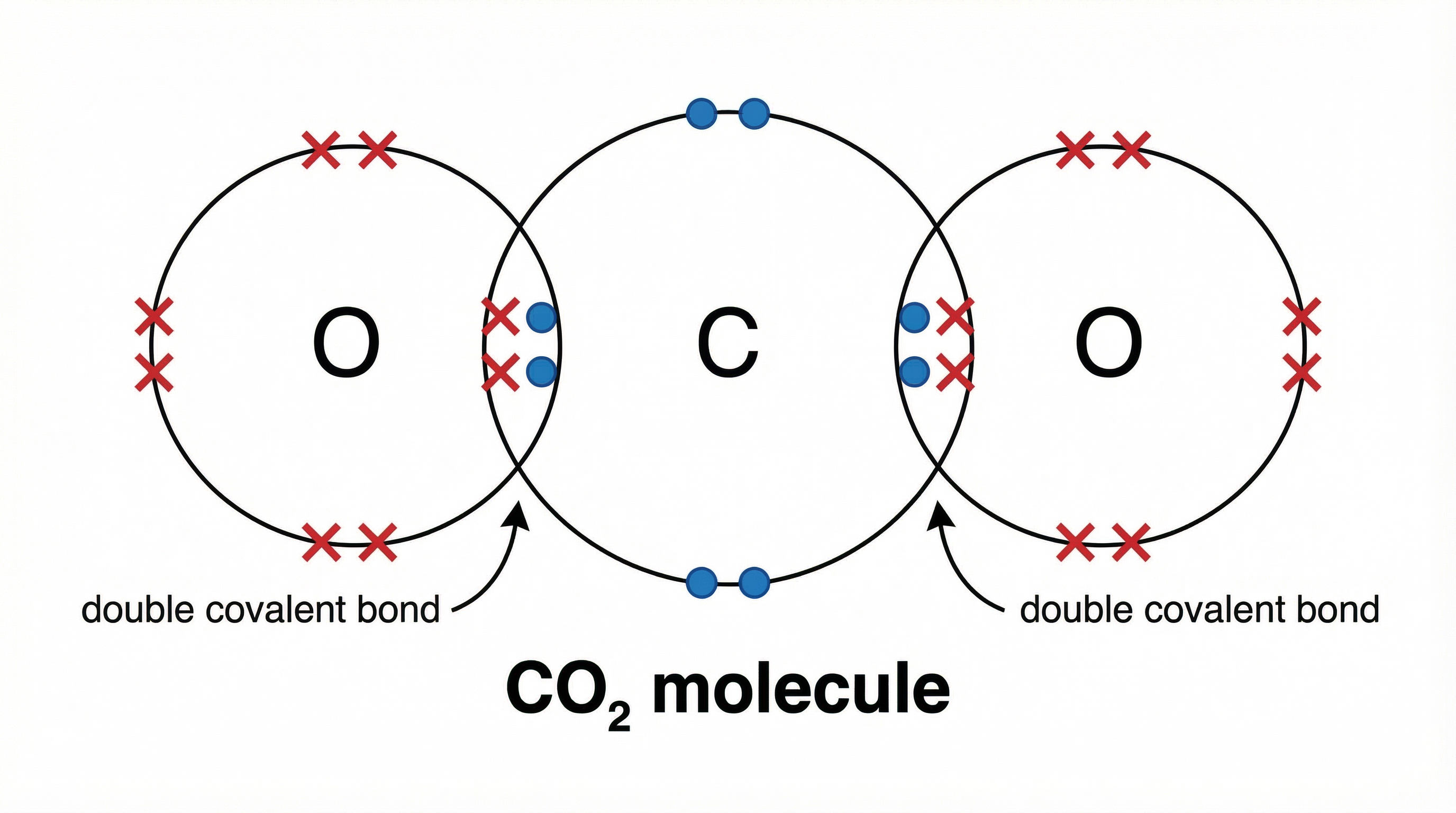
Concept 3: Double and Triple Covalent Bonds
Sometimes, atoms need to share more than one pair of electrons to achieve stability. This leads to double or triple bonds.
- A double bond involves two shared pairs of electrons (four electrons in total). Example: Oxygen gas (O₂).
- A triple bond involves three shared pairs of electrons (six electrons in total). Example: Nitrogen gas (N₂).
When drawing these, you simply place more electrons in the overlapping region. For carbon dioxide (CO₂), the carbon shares two pairs of electrons with each oxygen atom, forming two double bonds.
Mathematical/Scientific Relationships
There are no mathematical formulas for this topic, but the key relationship is understanding electron configuration based on the Periodic Table:
- The Group number tells you the number of electrons in the outer shell for main group elements.
- The Period number tells you the number of electron shells.
- To be stable, atoms (except for H, He, Li, Be) aim for 8 electrons in their outer shell (the Octet Rule).
Practical Applications
Understanding dot and cross diagrams helps explain the properties of everyday substances:
- Sodium Chloride (Table Salt): Its high melting point is due to the strong electrostatic forces between Na⁺ and Cl⁻ ions, which require a lot of energy to overcome.
- Water (H₂O): Its existence as a liquid at room temperature is due to the forces between its simple covalent molecules.
- Diamond vs Graphite: Both are made of carbon atoms. Diamond has a giant covalent structure with each carbon forming four strong covalent bonds, making it incredibly hard. Graphite has layers of covalently bonded carbon atoms with weak forces between the layers, allowing them to slide, which is why it's used in pencils.
