Study Notes
Overview
Electrolysis is a cornerstone of industrial chemistry and a topic that examiners frequently use to test candidates' fundamental understanding of chemical principles. For Edexcel GCSE Chemistry (1.15), mastering the electrolysis of molten compounds involves understanding how electricity can decompose a substance. It requires a firm grasp of ionic bonding, the states of matter, and the principles of oxidation and reduction (redox). In essence, you are using electrical energy to drive a non-spontaneous chemical reaction, breaking down a stable compound into its constituent elements. This process is vital for extracting reactive metals like aluminium and sodium from their ores, which cannot be extracted by simpler methods like reduction with carbon. Exam questions often focus on explaining the mechanism of conduction, predicting the products at each electrode, and, for Higher Tier candidates, constructing balanced half-equations. A typical 6-mark question might ask you to describe the entire process for a given molten salt, from explaining why it conducts to identifying the products and the reactions occurring at the cathode and anode.
Key Concepts
Concept 1: The Role of Ions in Electrical Conduction
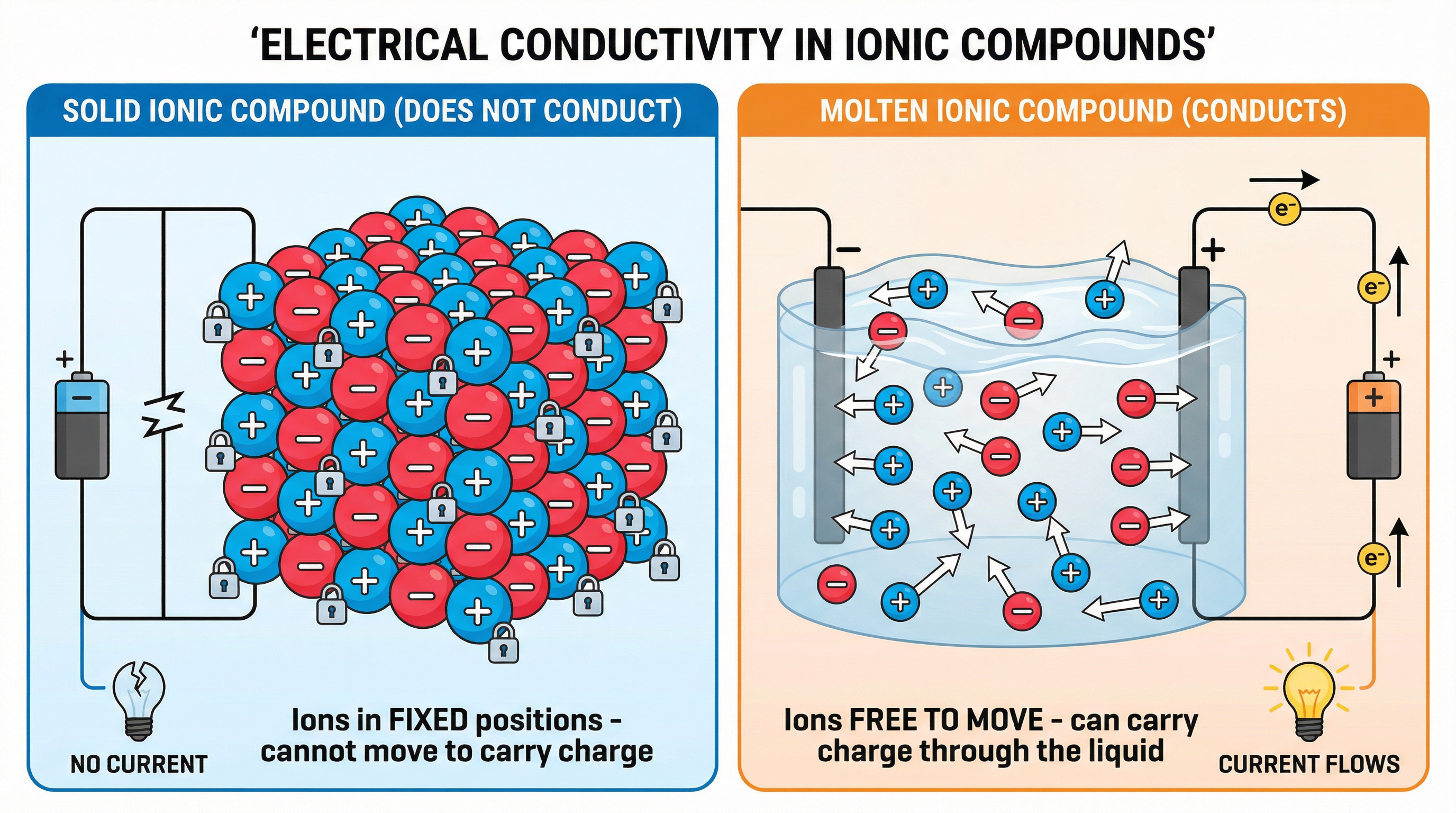
One of the most fundamental concepts, and a frequent source of marks, is explaining how an ionic compound conducts electricity. In its solid state, an ionic compound like lead(II) bromide forms a giant ionic lattice. The positive (cation) and negative (anion) ions are held in fixed positions by strong electrostatic forces of attraction. They can vibrate, but they cannot move from place to place. Because electrical current is the flow of charge, and these charged ions cannot move, the solid does not conduct electricity.
However, when the compound is heated until it melts, a crucial change occurs. The energy supplied overcomes the strong forces holding the lattice together, and the ions become free to move throughout the liquid. These mobile charge carriers are now able to travel towards the electrodes and complete the electrical circuit. It is essential to use the precise terminology: ions are free to move and carry charge. Stating that 'electrons move through the electrolyte' is a common and costly mistake.
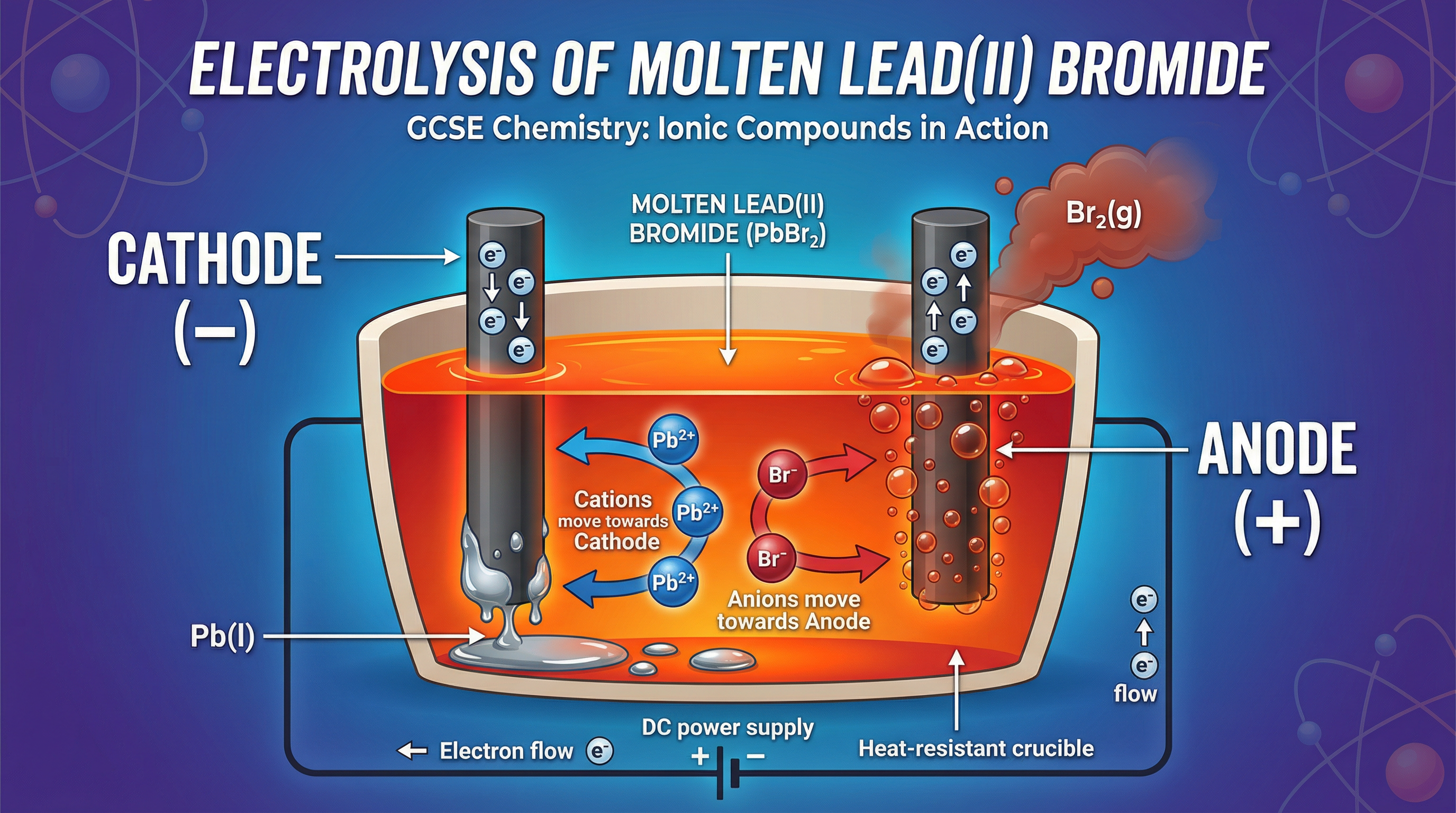
Concept 2: The Electrolytic Cell and Electrode Polarity
An electrolytic cell consists of two electrodes, a power supply, and the electrolyte (the molten ionic compound). The electrodes are typically made of an inert material like graphite, which won't react with the products.
- Cathode: The negative electrode. It is connected to the negative terminal of the power supply.
- Anode: The positive electrode. It is connected to the positive terminal of the power supply.
A simple mnemonic to remember this is PANIC: Positive Anode, Negative Is Cathode. In the molten electrolyte, the positively charged cations are attracted to the negative cathode. The negatively charged anions are attracted to the positive anode. This movement of ions constitutes the electric current within the electrolyte.
Concept 3: Redox Reactions at the Electrodes
Electrolysis is a redox process, meaning it involves both reduction and oxidation.
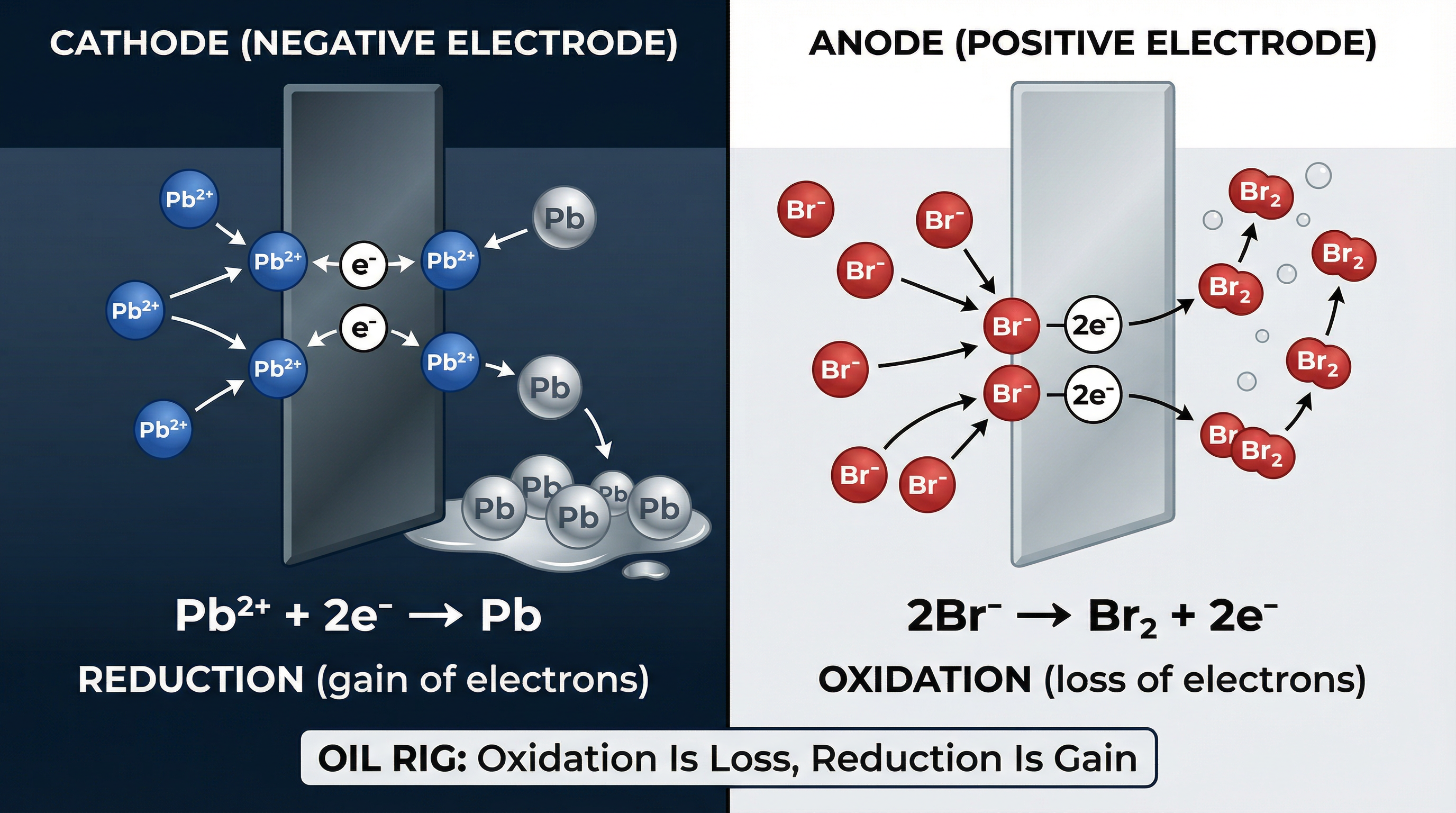
- Reduction at the Cathode: Cations (positive ions) move to the negative cathode. Here, they gain electrons and are reduced. For example, a lead ion gains two electrons to become a lead atom: Pb²⁺ + 2e⁻ → Pb(l).
- Oxidation at the Anode: Anions (negative ions) move to the positive anode. Here, they lose electrons and are oxidised. For example, two bromide ions lose one electron each to form a bromine molecule: 2Br⁻ → Br₂(g) + 2e⁻.
A way to remember this is OIL RIG: Oxidation Is Loss (of electrons), Reduction Is Gain (of electrons).
Mathematical/Scientific Relationships
Half-Equations (Higher Tier Only)
Half-equations are used to show the process of electron loss or gain at each electrode. They must be balanced for both atoms and charge.
Rules for writing half-equations:
- Identify the ion moving to the electrode.
- Write the formula of the product formed.
- Balance the atoms. For non-metal gases like chlorine or bromine, remember they are diatomic (Cl₂, Br₂).
- Balance the charge by adding electrons (e⁻) to the more positive side.
Example: Molten Aluminium Oxide (Al₂O₃)
- Ions present: Al³⁺ and O²⁻
- At the Cathode (-): Al³⁺ + 3e⁻ → Al (Reduction)
- At the Anode (+): 2O²⁻ → O₂ + 4e⁻ (Oxidation)
Practical Applications
The most significant industrial application of electrolysis of molten compounds is the extraction of reactive metals. The Hall-Héroult process is used to extract aluminium from its ore, bauxite. Bauxite is purified to get aluminium oxide (Al₂O₃), which is then dissolved in molten cryolite to lower its melting point from over 2000°C to around 950°C, saving huge amounts of energy. Electrolysis then separates the aluminium oxide into aluminium metal (at the cathode) and oxygen gas (at the anode). This process is a required topic and demonstrates the large-scale importance of the principles learned here.