Study Notes
Overview
Welcome to the essential guide for Edexcel GCSE Chemistry Topic 2.9: Fuels. This topic is a cornerstone of organic chemistry and is frequently tested in exams. It explores how we obtain useful fuels from crude oil, the relationship between the size of hydrocarbon molecules and their properties, and the environmental consequences of burning these fuels. A strong grasp of this area is crucial as it links directly to concepts of bonding, energy changes, and rates of reaction. Examiners often use this topic to assess all three Assessment Objectives (AOs), from recalling facts (AO1) and applying knowledge (AO2) to evaluating information (AO3), particularly in 6-mark questions comparing different fuels.
Key Concepts
Concept 1: Crude Oil and Fractional Distillation
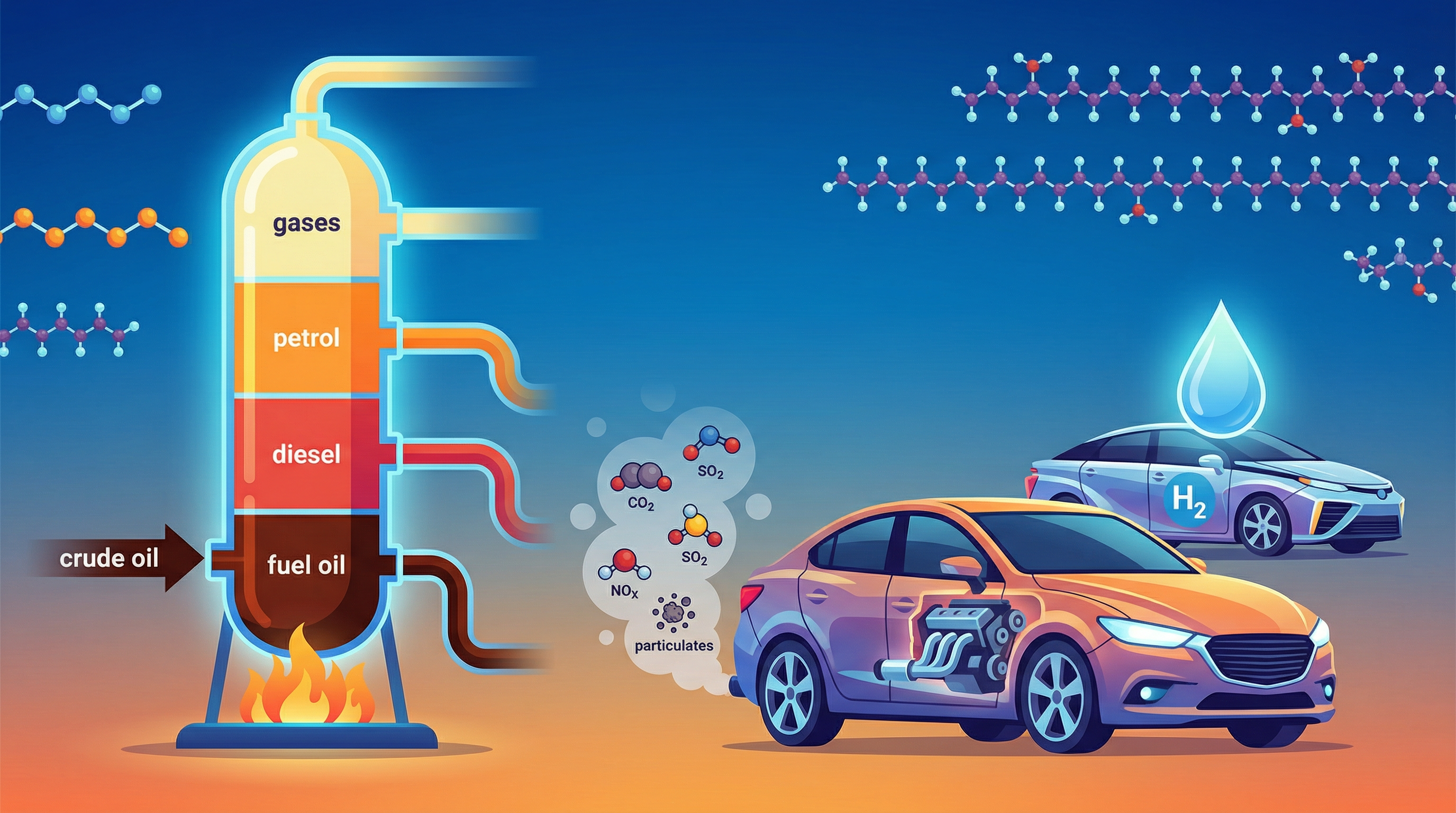
Crude oil is a finite resource found in rocks. It is a complex mixture of thousands of different hydrocarbons – molecules containing only carbon and hydrogen atoms. Because it's a mixture, the different hydrocarbons are not chemically bonded to each other and can be separated based on their physical properties. The process used to do this is fractional distillation.
The Process:
- Heating: Crude oil is heated to a high temperature (around 350-400°C), causing most of the hydrocarbons to evaporate and turn into a gas (vaporise). This hot mixture of liquid and vapour is pumped into the bottom of a fractionating column.
- Temperature Gradient: The column has a temperature gradient – it is very hot at the bottom and gradually gets cooler towards the top.
- Rising and Condensing: The hot hydrocarbon vapours rise up the column. As they rise, they cool down. Different hydrocarbons have different boiling points, so they condense (turn back into a liquid) at different levels.
- Long-chain hydrocarbons: These have strong intermolecular forces of attraction between the molecules. These forces require a lot of energy to overcome, so they have high boiling points. They condense at the hot lower levels of the column.
- Short-chain hydrocarbons: These have weak intermolecular forces. Less energy is needed to overcome these forces, so they have low boiling points. They continue to rise to the cooler upper levels of the column before condensing.
- Collection: The condensed liquids (fractions) are collected on trays at their respective levels and piped off. Very short-chain hydrocarbons with very low boiling points don't condense and are removed as gases from the top of the column.
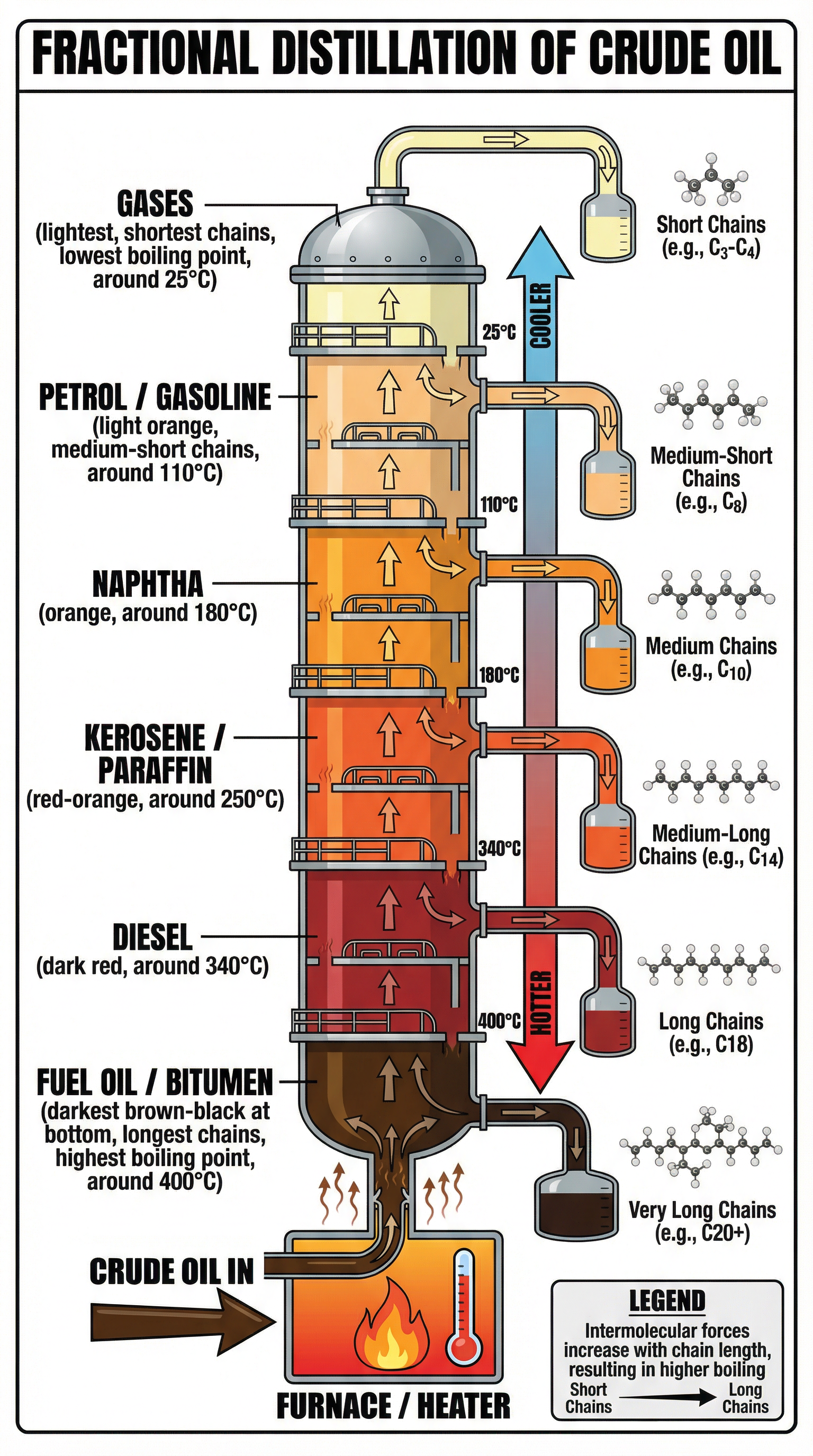
**Key Properties of Fractions:**As the size of the hydrocarbon molecule (carbon chain length) increases:
- Boiling Point: Increases (due to stronger intermolecular forces).
- Viscosity: Increases (the liquid becomes thicker and flows less easily).
- Flammability: Decreases (they become harder to ignite).
- Colour: The liquid becomes darker and dirtier.
Concept 2: Combustion of Fuels
Combustion is the scientific term for burning. It is an exothermic reaction where a fuel reacts with oxygen to release energy. The products of combustion depend on the amount of oxygen available.
**Complete Combustion:**This occurs when there is a plentiful supply of oxygen. All the fuel burns. For hydrocarbons, the only products are carbon dioxide (CO2) and water (H2O).
General Equation: Hydrocarbon + Oxygen → Carbon Dioxide + Water
Example (Methane): CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)
**Incomplete Combustion:**This occurs when the supply of oxygen is limited. Not all the fuel can burn completely. The products can include carbon monoxide (CO), a toxic gas, and/or carbon (C) in the form of soot or particulates, as well as water.
Example (Methane): 2CH4(g) + 3O2(g) → 2CO(g) + 4H2O(l)
Example (Methane): CH4(g) + O2(g) → C(s) + 2H2O(l)
Concept 3: Pollutants from Combustion
Burning fossil fuels releases several harmful pollutants into the atmosphere. Examiners expect you to know where they come from and the problems they cause.
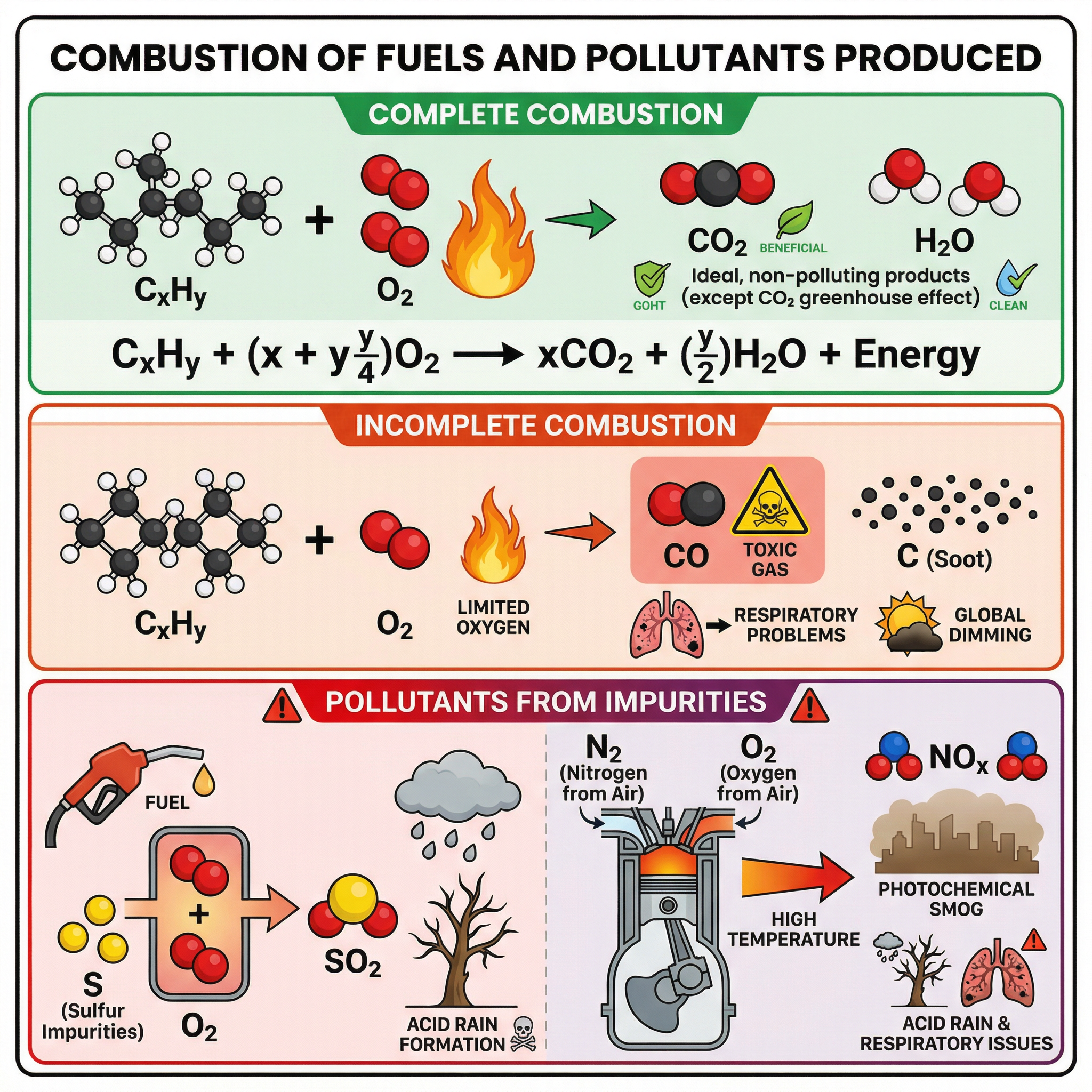
- Sulfur Dioxide (SO2): Most fossil fuels contain sulfur impurities. When the fuel is burned, this sulfur reacts with oxygen to form sulfur dioxide. SO2 dissolves in water droplets in clouds to form sulfuric acid, which then falls as acid rain. Acid rain damages limestone buildings, corrodes metal structures, and harms ecosystems by making rivers and soil too acidic.
- Nitrogen Oxides (NOx): The air is about 78% nitrogen. At the very high temperatures and pressures inside a car engine, nitrogen and oxygen from the air can react together to form various nitrogen oxides. These also contribute to acid rain and can cause photochemical smog and respiratory problems.
- Carbon Monoxide (CO): As seen above, this is a product of incomplete combustion. It is a toxic gas because it is readily absorbed by red blood cells in place of oxygen, starving the body of oxygen.
- Particulates (Soot): These are tiny solid particles of carbon produced during incomplete combustion. They can cause respiratory problems and contribute to global dimming by reflecting sunlight back into space.
Concept 4: Hydrogen as an Alternative Fuel
Hydrogen is often proposed as a clean alternative to fossil fuels. It can be used in fuel cells to power electric vehicles.
**Combustion of Hydrogen:**Hydrogen burns in oxygen to produce only water. This means no CO2, SO2, or carbon particulates are produced.
Equation: 2H2(g) + O2(g) → 2H2O(l)
Evaluation (A key AO3 skill):
| Advantages of Hydrogen | Disadvantages of Hydrogen |
|---|---|
| Only produces water (no CO2, so no contribution to global warming). | Most hydrogen is currently produced from fossil fuels, which releases CO2. |
| Releases more energy per kilogram than petrol. | Difficult and dangerous to store; it is a gas under high pressure or a liquid at very low temperatures. |
| Fuel cells are more efficient than internal combustion engines. | Lack of a widespread hydrogen refueling infrastructure. |
Mathematical/Scientific Relationships
There are no complex formulas to memorise for this topic, but you must be able to write and balance symbol equations for combustion reactions. The key is to balance the atoms in this order: Carbon, then Hydrogen, then Oxygen (CHO).
Example: Balancing the complete combustion of propane (C3H8)
- Start with the unbalanced equation: C3H8 + O2 → CO2 + H2O
- Balance Carbon: There are 3 carbons on the left, so we need 3 CO2 on the right.
C3H8 + O2 → 3CO2 + H2O - Balance Hydrogen: There are 8 hydrogens on the left, so we need 4 H2O on the right (4 x 2 = 8).
C3H8 + O2 → 3CO2 + 4H2O - Balance Oxygen: Now count the oxygens on the right. There are (3 x 2) + 4 = 10 oxygens. So we need 5 O2 on the left.
Final Balanced Equation: C3H8 + 5O2 → 3CO2 + 4H2O
Practical Applications
This topic is all about real-world applications. The fractions from crude oil are essential for modern life:
- Gases: Domestic heating and cooking (LPG).
- Petrol (Gasoline): Fuel for cars.
- Kerosene: Jet fuel for aircraft, paraffin for lamps.
- Diesel: Fuel for lorries, buses, and trains.
- Fuel Oil: Fuel for ships and power stations.
- Bitumen: Road surfacing and roofing.
This topic does not have a specific required practical, but the principles of distillation are covered in Required Practical 15 (Separating substances).