Study Notes
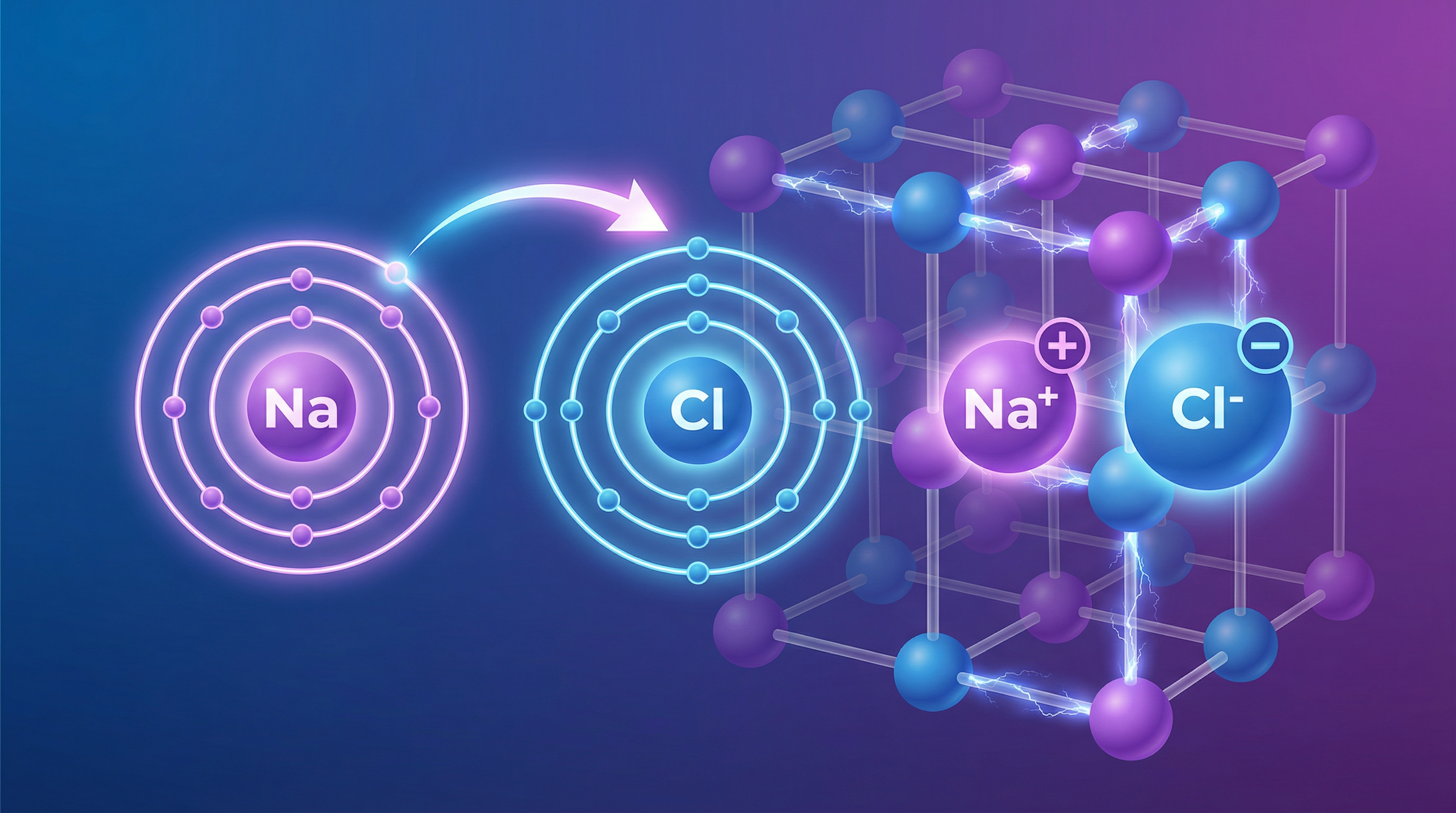
Overview
Welcome to the world of ionic bonding, a cornerstone of your Edexcel GCSE Chemistry course (Topic 1.6). This topic explores how metal and non-metal atoms achieve stability by transferring electrons, transforming into charged particles called ions. The powerful electrostatic forces between these ions create giant, rigid structures with unique properties that you need to be able to explain with scientific precision. Understanding this process is not just about drawing diagrams; it's about linking the sub-atomic world of electrons to the macroscopic properties we can observe, like high melting points and electrical conductivity. Examiners frequently test this area with structured questions that require you to build a logical argument, so mastering the key definitions and explanatory steps is essential for securing those higher-level marks.
Key Concepts
Concept 1: Formation of Ions by Electron Transfer
Atoms are neutral because they have an equal number of positive protons and negative electrons. However, atoms are most stable when they have a full outer shell of electrons, like the noble gases in Group 0. To achieve this, metal atoms (from Groups 1, 2, and 3) tend to lose electrons, forming positive ions (cations). Non-metal atoms (from Groups 6 and 7) tend to gain electrons, forming negative ions (anions). This process is called electron transfer.
Example: Sodium (Na) is in Group 1 with the electron configuration 2.8.1. It loses its single outer electron to become a sodium ion (Na+) with a stable configuration of 2.8. Chlorine (Cl) is in Group 7 (2.8.7). It gains one electron to become a chloride ion (Cl-) with a stable configuration of 2.8.8. The electron is transferred from the sodium atom to the chlorine atom.
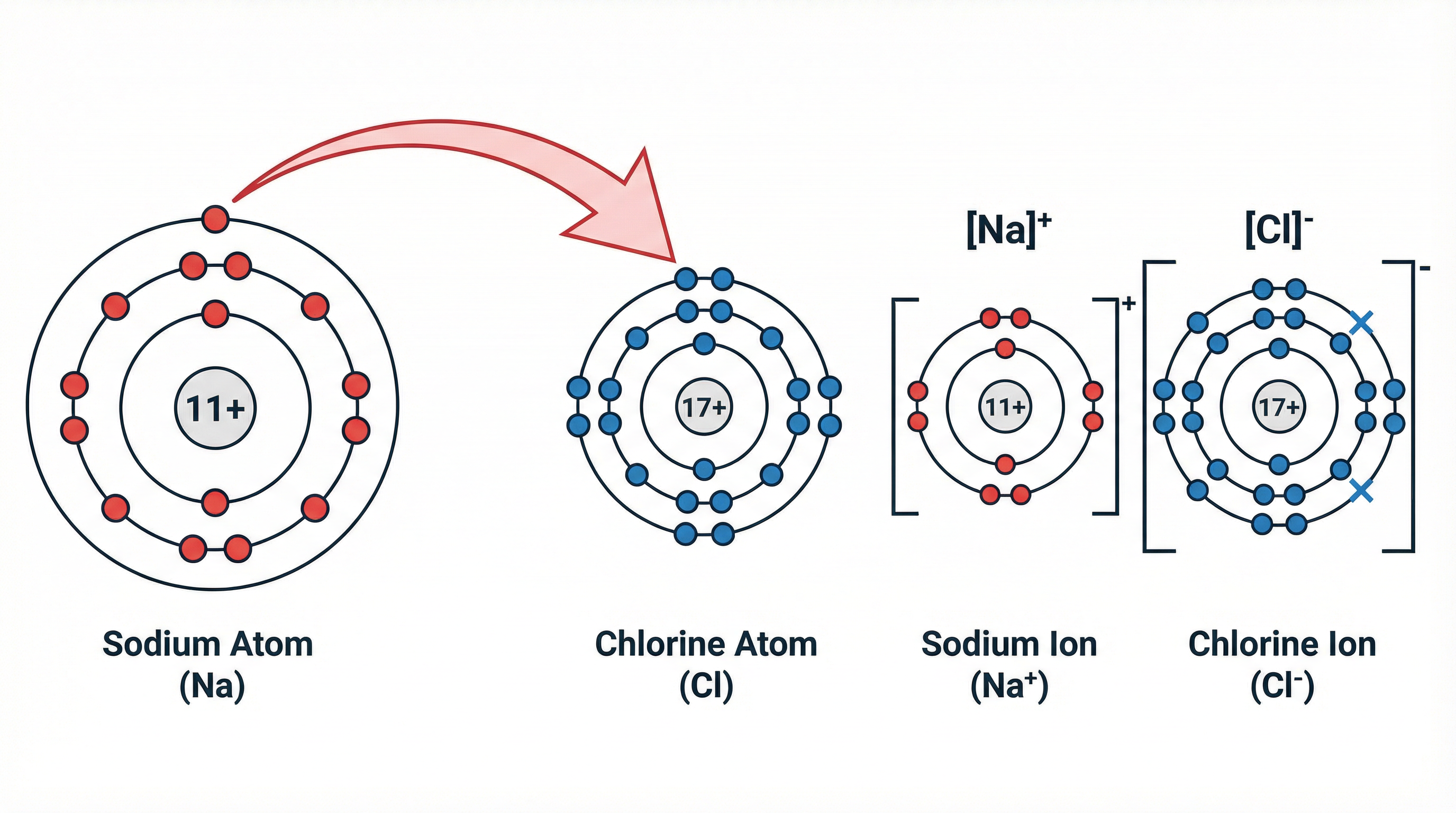
Concept 2: The Ionic Bond and the Giant Ionic Lattice
Once the oppositely charged ions are formed, they are strongly attracted to each other. The ionic bond is defined as the strong electrostatic force of attraction between oppositely charged ions. This is a fundamental definition you must learn. These forces are not just between one pair of ions; they act in all directions. This results in the formation of a giant ionic lattice, which is a regular, repeating three-dimensional arrangement of ions. In a sodium chloride lattice, for instance, every Na+ ion is surrounded by six Cl- ions, and every Cl- ion is surrounded by six Na+ ions. It is crucial to remember that ionic compounds do not form simple molecules; they form these vast, ordered structures.
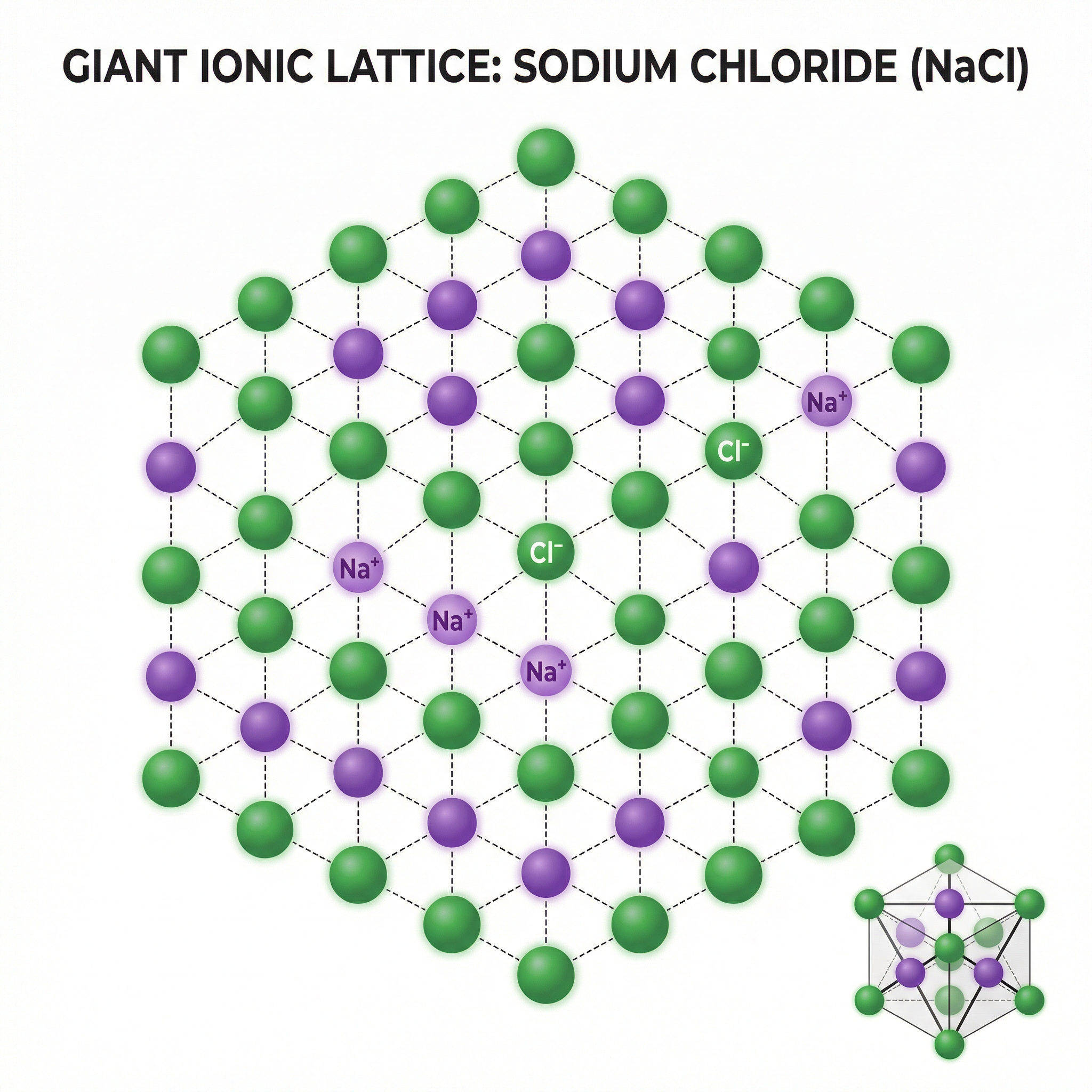
Concept 3: Physical Properties of Ionic Compounds
The giant ionic lattice structure directly determines the physical properties of ionic compounds:
- High Melting and Boiling Points: A large amount of thermal energy is needed to overcome the strong electrostatic forces of attraction between the ions in the lattice. This is why ionic compounds are solids at room temperature and have very high melting points. You must mention both the 'strong forces' and the 'large amount of energy' to gain full credit in an exam.
- Electrical Conductivity: For a substance to conduct electricity, it must contain charged particles that are free to move. In a solid ionic compound, the ions are charged, but they are held in fixed positions within the lattice and cannot move. Therefore, solid ionic compounds do not conduct electricity. However, when an ionic compound is molten (melted) or dissolved in water (aqueous solution), the ions are no longer fixed and are free to move and carry charge. Therefore, molten and aqueous ionic compounds do conduct electricity. It is the ions that move, not the electrons.
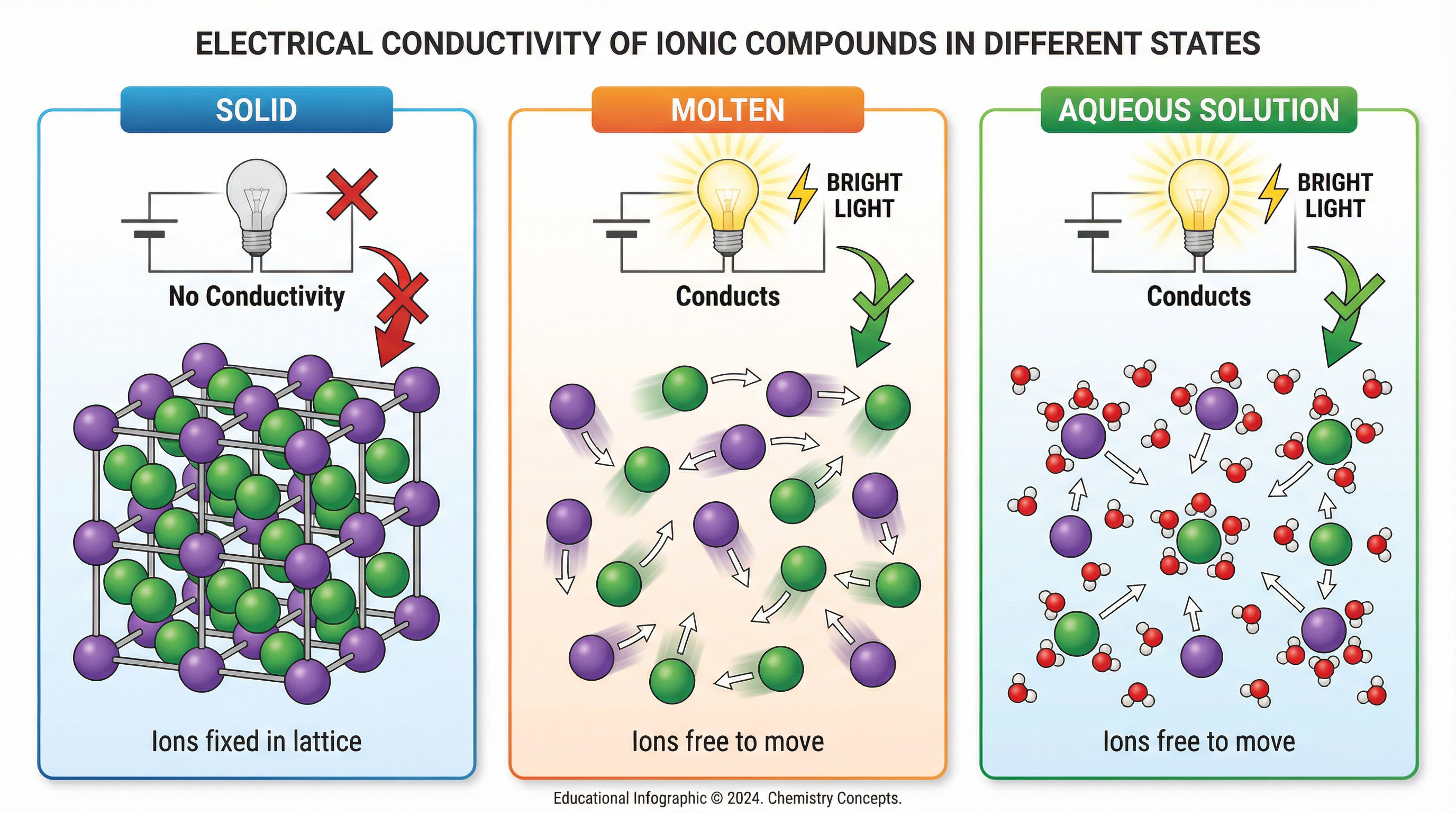
Mathematical/Scientific Relationships
There are no complex mathematical formulas in this topic at GCSE level. The key relationships are conceptual:
- Charge of an ion: The charge on a simple ion can be predicted from its group in the periodic table.
- Group 1 metals form +1 ions (e.g., Na+, K+).
- Group 2 metals form +2 ions (e.g., Mg2+, Ca2+).
- Group 6 non-metals form -2 ions (e.g., O2-, S2-).
- Group 7 non-metals form -1 ions (e.g., F-, Cl-).
- Overall Charge of a Compound: The sum of the positive and negative charges in an ionic compound must equal zero. This allows you to deduce the formula. For example, to balance the 2+ charge of a magnesium ion (Mg2+), you need two chloride ions (Cl-), giving the formula MgCl2.
Practical Applications
Ionic bonding is fundamental to many real-world applications:
- Table Salt (NaCl): The classic example of an ionic compound, used for flavouring and preserving food.
- Ceramics (e.g., Magnesium Oxide, MgO): The high melting points of ionic compounds like magnesium oxide make them suitable for furnace linings and other high-temperature applications.
- Electrolysis: The conductivity of molten or aqueous ionic compounds is exploited in electrolysis to extract reactive metals (like aluminium from aluminium oxide) or produce other useful chemicals (like chlorine from sodium chloride solution).
- Batteries: The movement of ions is the basis for how many batteries and electrochemical cells generate an electric current.
