Study Notes
Overview
Welcome to the foundational topic of your GCSE Chemistry journey: Atomic Structure and the Periodic Table (AQA 2.1). This unit is the bedrock upon which all other chemical concepts are built. Understanding the nature of atoms is not just about memorising particles; it's about grasping why elements behave the way they do. From the simple definition of an atom to the complex reasons behind periodic trends, mastering this topic is crucial for exam success. In the exam, you can expect a mix of short-answer definition questions, calculation-based problems (like finding the number of neutrons or relative atomic mass), and longer 6-mark questions, often asking you to compare atomic models or explain reactivity trends. This guide will equip you with the knowledge, exam technique, and memory aids to tackle them all confidently.
{{asset:atomic_structure_and_the_periodic_table_podcast.mp3}}
Key Concepts
1. The Structure of the Atom
At the heart of chemistry is the atom, the smallest unit of an element. Atoms are composed of a central nucleus containing protons and neutrons, with electrons orbiting this nucleus in specific energy levels or shells. The nucleus is incredibly small and dense compared to the overall size of the atom, which is mostly empty space. For your exam, you must know the relative charges and masses of these subatomic particles.
| Particle | Relative Mass | Relative Charge |
|---|---|---|
| Proton | 1 | +1 |
| Neutron | 1 | 0 |
| Electron | ~0 (1/1836) | -1 |
- Atomic Number (Z): The number of protons in the nucleus. This number defines the element. For a neutral atom, it also equals the number of electrons.
- Mass Number (A): The total number of protons and neutrons in the nucleus.
Example: A sodium atom (Na) has an atomic number of 11 and a mass number of 23. This means it has:
- 11 protons
- 11 electrons (in a neutral atom)
- 23 - 11 = 12 neutrons
2. The Evolution of the Atomic Model
The way we picture the atom has changed over time as new evidence emerged. A common 6-mark question asks you to describe this journey. Credit is given for a chronological account linking models to the scientists and experiments that defined them.
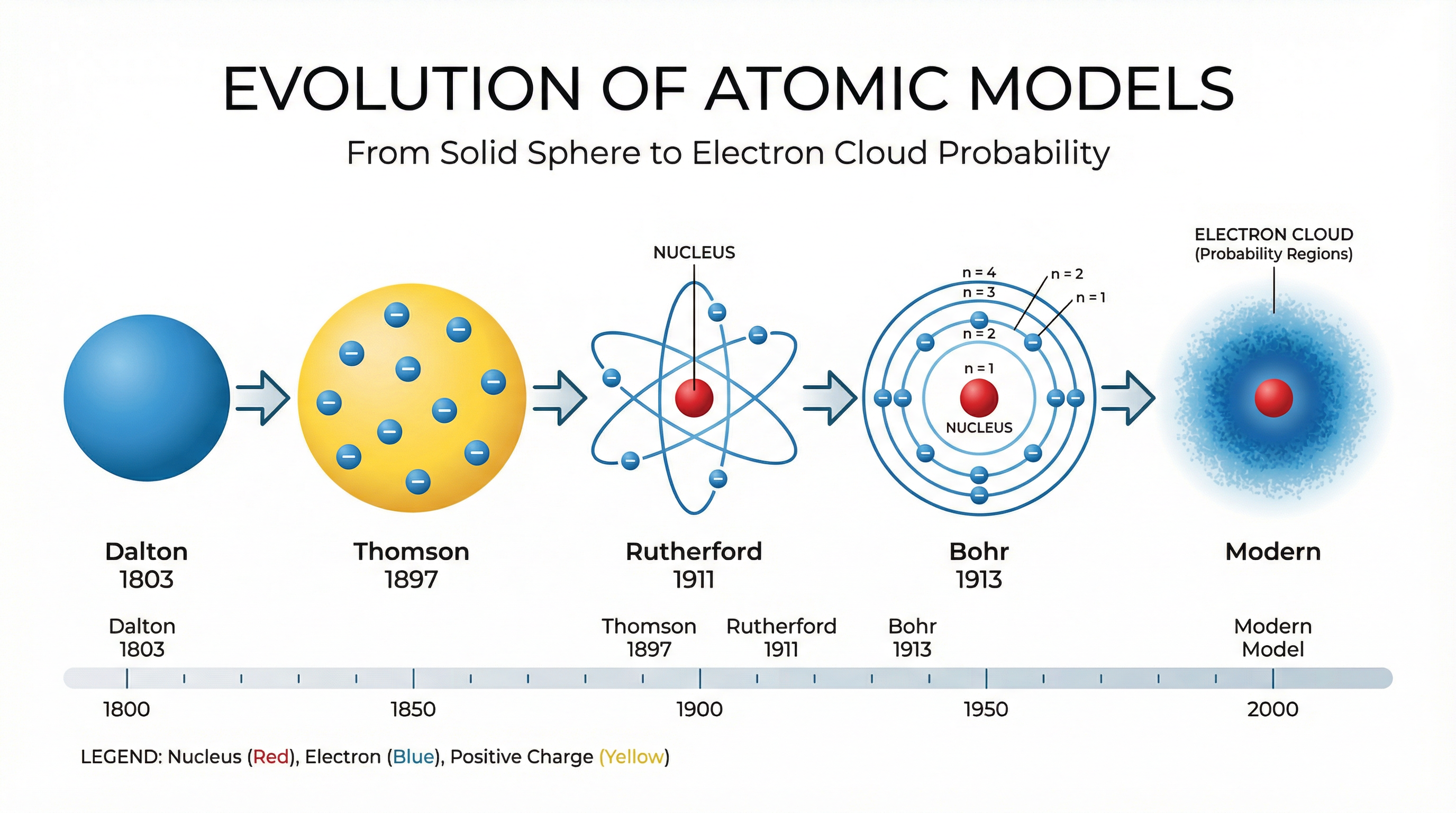
- John Dalton (1803): Proposed that atoms were tiny, indivisible solid spheres.
- J.J. Thomson (1897): After discovering the electron, he suggested the Plum Pudding Model. The atom was a ball of positive charge with negative electrons embedded within it.
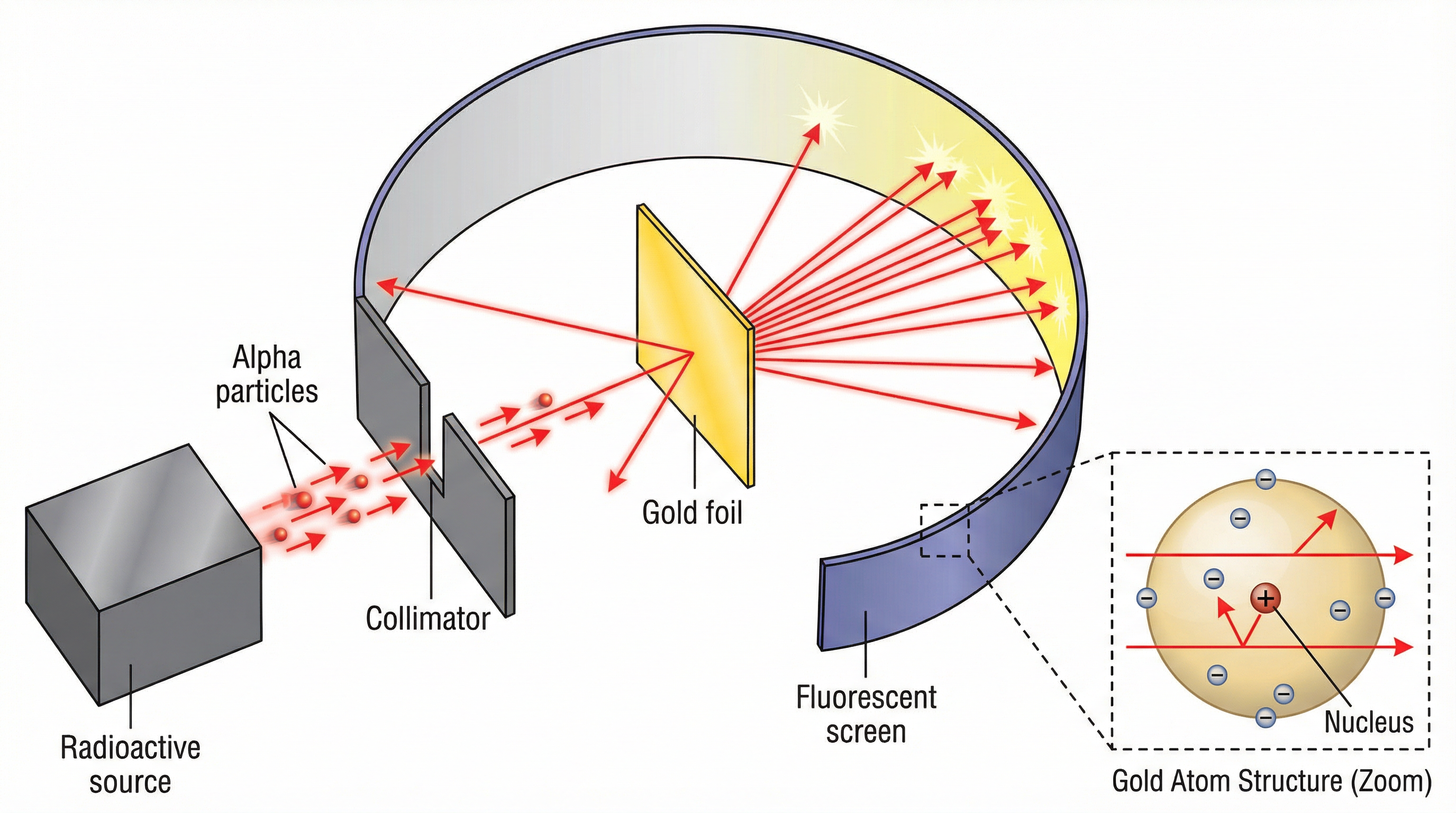
- Ernest Rutherford (1911): His famous Alpha Scattering Experiment disproved the Plum Pudding model. By firing alpha particles at thin gold foil, he made two key observations:
- Most alpha particles passed straight through, showing the atom is mostly empty space.
- A tiny number were deflected at large angles, showing a small, dense, positively charged nucleus must exist.
This led to the nuclear model.
- Niels Bohr (1913): He adapted the nuclear model by suggesting electrons orbit the nucleus in fixed energy shells.
- James Chadwick (1932): Provided the evidence for the existence of the neutron, completing our modern understanding of the subatomic particles in the nucleus.
3. Isotopes and Relative Atomic Mass
Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. Because they have the same number of electrons, isotopes have the same chemical properties.
Example: Carbon-12 (6 protons, 6 neutrons) and Carbon-14 (6 protons, 8 neutrons) are isotopes of carbon.
Relative Atomic Mass (Ar) is the weighted mean mass of an atom of an element compared with 1/12th of the mass of an atom of carbon-12. To calculate it, you use the following formula:
Ar = [ (isotope mass 1 x % abundance 1) + (isotope mass 2 x % abundance 2) ] / 100
This is a frequent calculation question, so ensure you can apply this formula correctly.
4. The Periodic Table
The periodic table arranges elements in order of increasing atomic number. The columns are called groups, and the rows are called periods.
- Groups: Elements in the same group have the same number of electrons in their outer shell. This gives them similar chemical properties.
- Periods: Elements in the same period have the same number of electron shells.
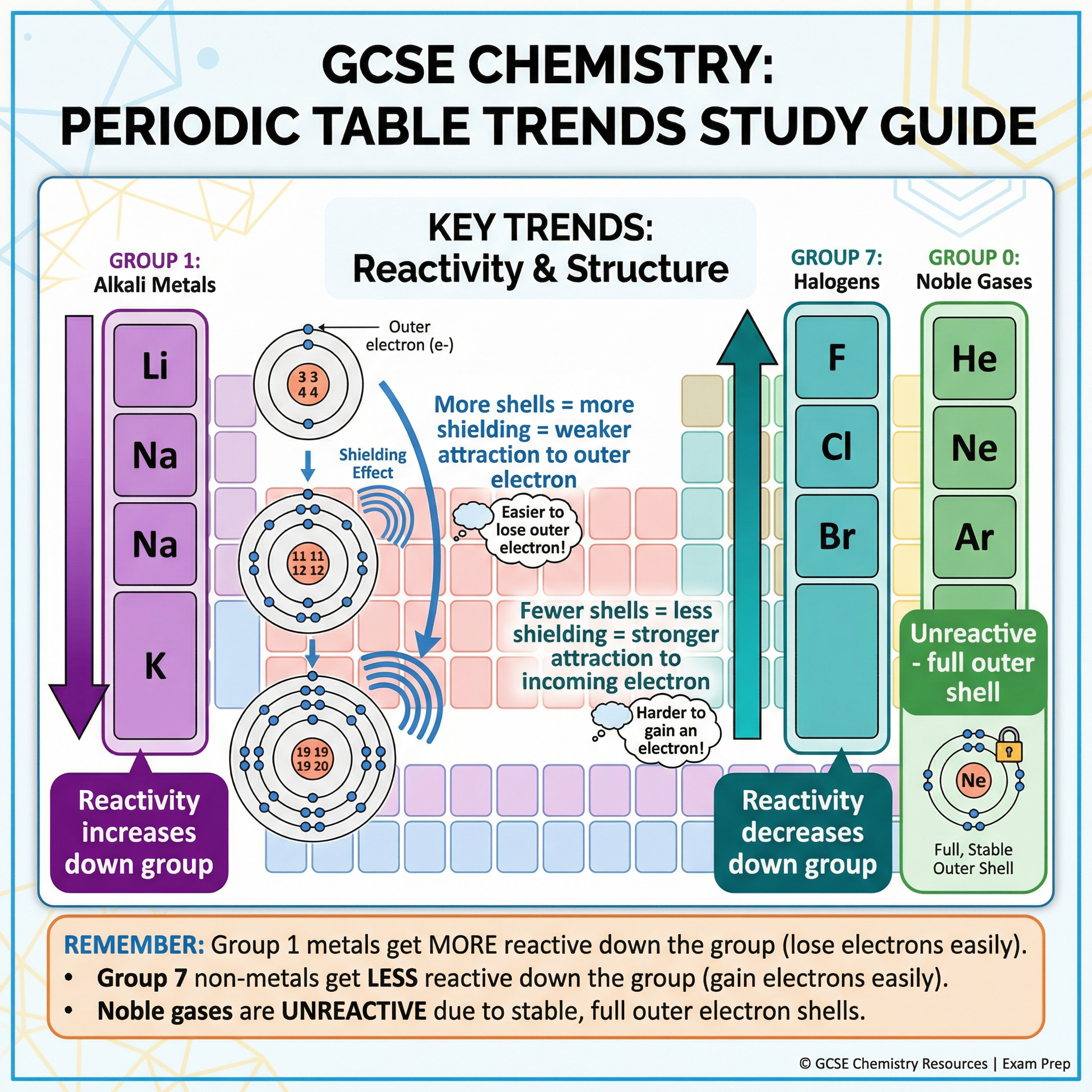
5. Group Trends
Examiners frequently test your understanding of trends in Groups 1, 7, and 0.
-
Group 1 (Alkali Metals): Reactivity increases down the group. As you go down the group:
- The atoms get larger.
- The outer electron is further from the nucleus.
- There is more shielding from the inner electron shells.
- The electrostatic attraction between the positive nucleus and the negative outer electron becomes weaker.
- The electron is more easily lost.
-
Group 7 (The Halogens): Reactivity decreases down the group. As you go down the group:
- The atoms get larger.
- The outer shell is further from the nucleus.
- There is more shielding from inner electrons.
- The electrostatic attraction for an incoming electron is weaker.
- An electron is less easily gained.
-
Group 0 (The Noble Gases): They are unreactive. This is because they have a full outer shell of electrons, which is a very stable electronic configuration. They do not need to lose, gain, or share electrons to become stable.
Mathematical/Scientific Relationships
- Calculating Neutrons:
Number of Neutrons = Mass Number (A) - Atomic Number (Z)(Must memorise) - Relative Atomic Mass (Ar):
Ar = [ (mass₁ x abundance₁) + (mass₂ x abundance₂) ] / 100(Given on formula sheet)
Practical Applications
Mixtures and Separation Techniques
This topic links to the required practical on separating mixtures. You need to know which technique to use based on the physical properties of the substances.
- Filtration: Separates an insoluble solid from a liquid (e.g., sand from water).
- Crystallisation/Evaporation: Separates a soluble solid from a solution (e.g., salt from water).
- Simple Distillation: Separates a liquid from a solution (e.g., pure water from salt water). This works because the substances have different boiling points.
- Fractional Distillation: Separates a mixture of liquids with different boiling points (e.g., ethanol from water).
- Chromatography: Separates different soluble, coloured substances from a mixture (e.g., inks). This works because different substances have different solubilities in the solvent and attractions to the paper.