Study Notes
Overview
Welcome to the fascinating world of pharmacology! This topic, Developing New Medicines, explores the rigorous journey from a promising chemical compound to a life-saving drug available at your local pharmacy. For your AQA GCSE Biology exam, you'll need to understand this process in detail, as it's a prime source for 4- and 6-mark questions that test your ability to describe a sequence accurately. This guide will walk you through the key stages: discovery, preclinical testing, and clinical trials, ensuring you can explain not just what happens, but why each step is so critical. We'll also link this to concepts like cell biology and disease, showing how different areas of your specification connect in the real world.
Key Concepts
Concept 1: Drug Discovery - From Nature to the Lab
Many of the most important medicines used today originated from living organisms. It is crucial for candidates to recall specific examples, as marks are frequently awarded for this knowledge.
- Digitalis: A drug used to strengthen the heartbeat, originally extracted from the foxglove plant. It's a classic example of a traditional remedy being scientifically developed.
- Aspirin: A common painkiller and anti-inflammatory drug, with its origins in the bark of the willow tree. Salicylic acid, the active ingredient, was used for centuries before being synthesised in the lab to reduce side effects.
- Penicillin: The first widely used antibiotic, discovered by Alexander Fleming in 1928. He observed that the Penicillium mould produced a substance that killed bacteria. This discovery revolutionised the treatment of bacterial infections.
While some drugs are still extracted, today many are synthesised by chemists in the pharmaceutical industry. These scientists may create variations of existing drugs or design new molecules from scratch to target specific diseases.
Concept 2: Preclinical Testing - Is it Safe?
Before any new drug can be tested on humans, it must undergo rigorous preclinical testing. This stage is all about assessing the drug's toxicity (safety). The primary goal is to identify any harmful side effects before exposing people to the drug.
The process involves testing on:
- Cells and Tissues: The drug is applied to cells and tissues grown in the laboratory to see how it affects them at a microscopic level.
- Live Animals: If the drug passes the first stage, it is then tested on live animals (often mice or rats) to observe its effects on a whole, living organism. This allows scientists to monitor for any adverse effects on the circulatory, nervous, and organ systems.
This stage is vital for establishing an initial safe dosage and predicting potential hazards. Only drugs that pass preclinical testing can proceed to the next phase.
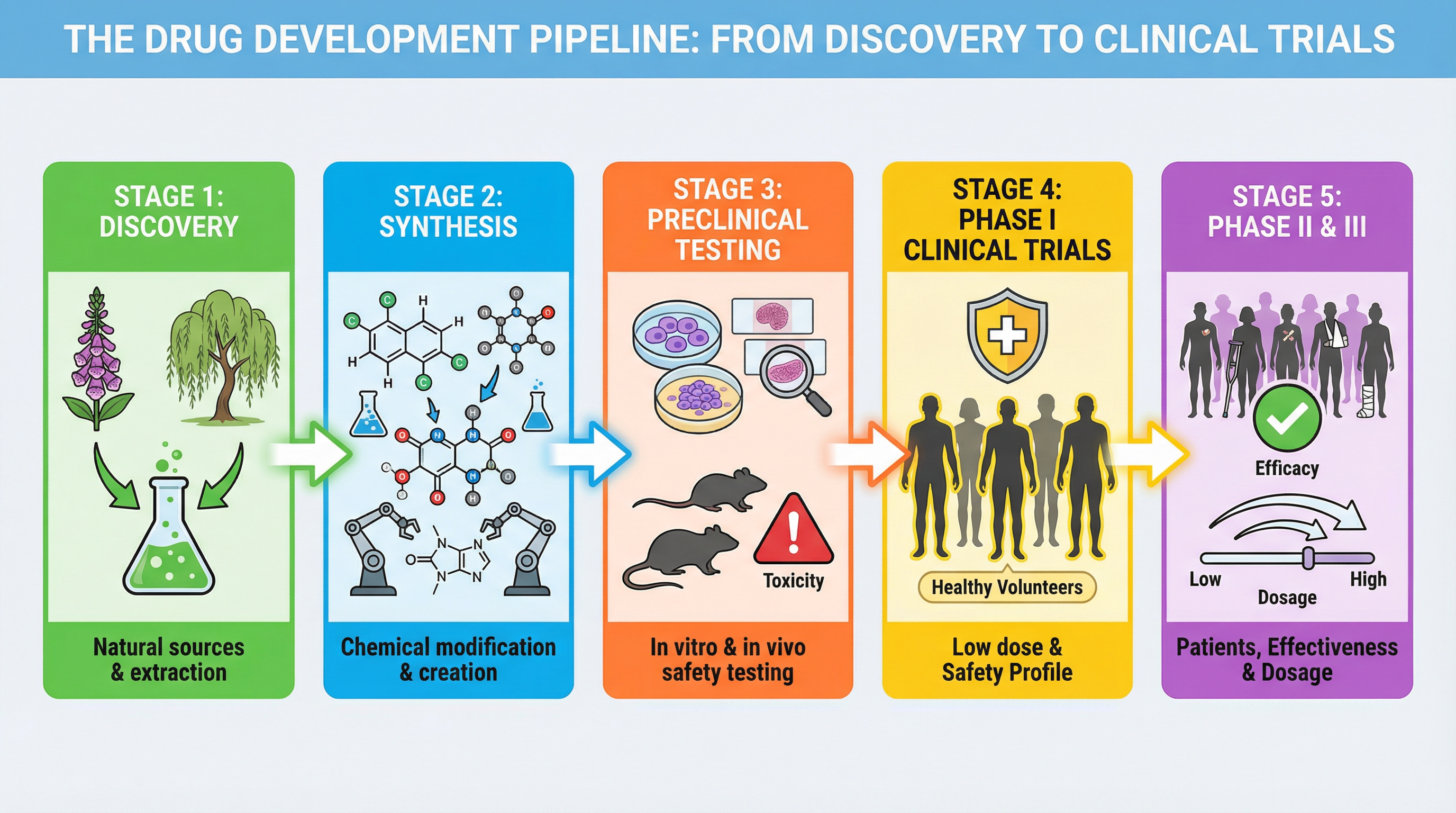
Concept 3: Clinical Trials - Testing in Humans
Clinical trials involve testing the drug on human volunteers and patients. This process is carefully structured into phases to ensure safety and gather reliable data on the drug's efficacy (effectiveness) and optimal dosage.
- Phase I: The drug is given to a small number of healthy volunteers. The primary aim is to confirm the drug's safety in humans, monitor for side effects, and determine a safe dosage range. Doses start very low and are gradually increased.
- Phase II: The drug is given to a larger group of patients with the target condition. This phase aims to determine the drug's efficacy – does it actually work in treating the illness? Dosage is also further refined.
- Phase III: The drug is tested on a much larger group of patients (often thousands) to confirm its effectiveness, monitor side effects, and compare it to existing treatments. This provides the robust data needed for the drug to be licensed for use.
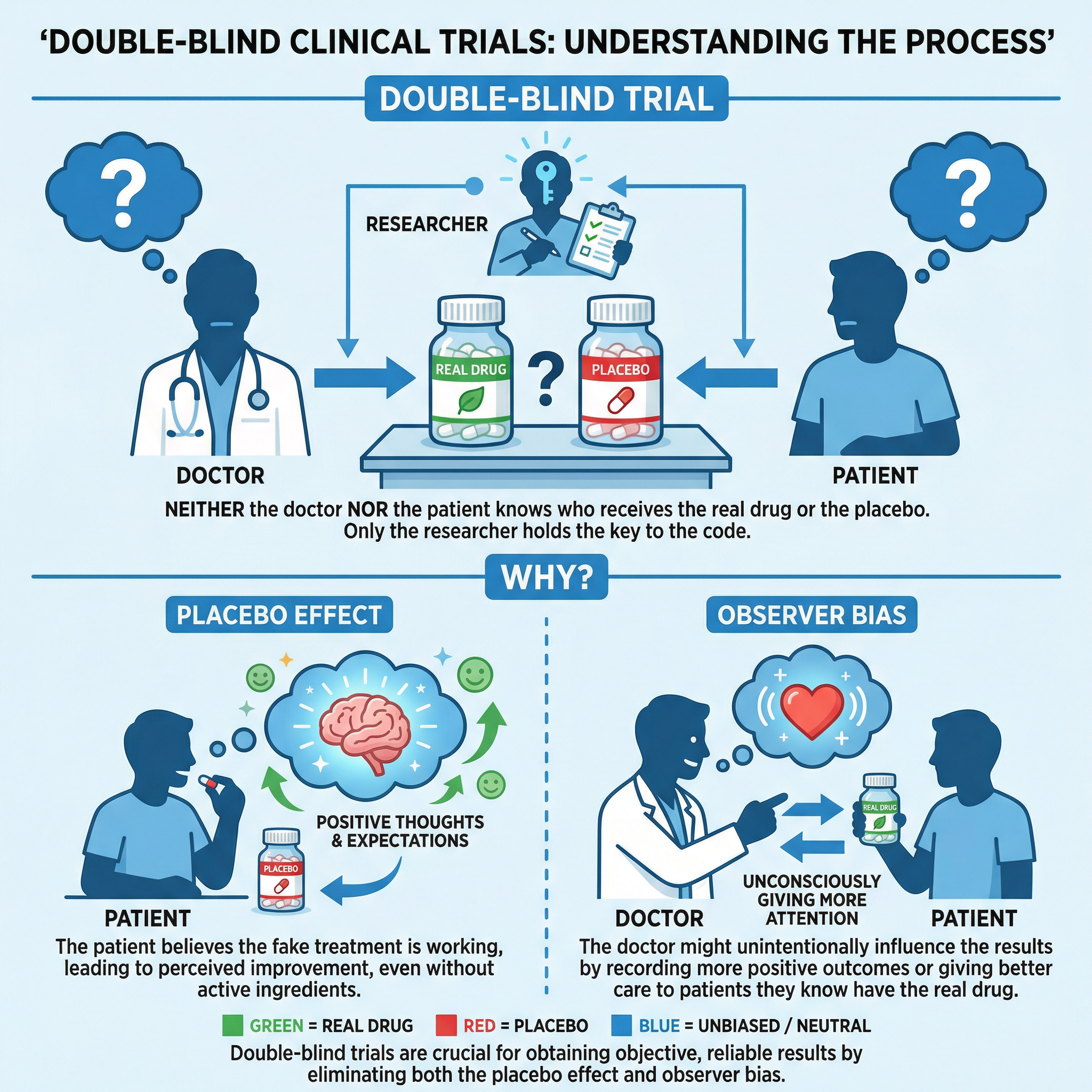
Concept 4: Double-Blind Trials and Placebos
To ensure the results of clinical trials are unbiased, the 'gold standard' method is the double-blind, placebo-controlled trial. This is a key concept that frequently appears in exams.
- Placebo: A substance that has no therapeutic effect, used as a control in testing new drugs. It is a 'dummy' drug that looks identical to the real one (e.g., a sugar pill).
- Double-Blind: In this type of trial, neither the patients nor the doctors know who is receiving the real drug and who is receiving the placebo. An independent researcher holds the key.
This method is crucial for eliminating two types of bias:
- The Placebo Effect: When a patient's symptoms improve simply because they believe they are receiving treatment, even if it's inactive.
- Observer Bias: When a doctor's expectations or hopes for a new drug unconsciously influence how they assess a patient's condition.
By using a double-blind design, scientists can be confident that any observed effects are due to the drug itself and not psychological factors.
Mathematical/Scientific Relationships
There are no specific mathematical formulas to memorise for this topic. However, candidates may be presented with data from clinical trials in tables or graphs. You will need to be able to:
- Calculate percentages (e.g., the percentage of patients who showed improvement).
- Interpret graphs showing the effect of a drug over time.
- Compare the effectiveness of a new drug with an existing drug or a placebo using provided data.
Practical Applications
This topic is directly linked to the development of all modern medicines, from antibiotics to cancer treatments. Understanding this process is fundamental to appreciating how medical science advances. While there isn't a specific 'required practical' for this topic, the principles of fair testing, controlling variables, and avoiding bias are central to all practical work in GCSE Biology.