Study Notes
Overview
Enzymes are the unsung heroes of the biological world. They are highly specific protein molecules that act as biological catalysts, speeding up the chemical reactions necessary for life without being used up in the process. For your WJEC GCSE Biology exam, a thorough understanding of enzymes is non-negotiable. This topic, 1.6, frequently appears in both Foundation and Higher tier papers, often in the form of data interpretation questions (AO2) and longer, structured explanations (AO1). You will be expected to explain the ‘lock and key’ hypothesis with precision, analyse graphs showing the effects of temperature and pH, and apply your knowledge to practical contexts. This topic forms a cornerstone of biology, linking directly to digestion, respiration, and photosynthesis, making it a vital synoptic link that examiners love to test.
Key Concepts
Concept 1: The Nature of Enzymes
At its core, an enzyme is a biological catalyst made of protein. Let’s break that down for marks:
- Biological: They are produced by and found within living organisms.
- Catalyst: They increase the rate of a chemical reaction but are not changed or consumed by it. This means one enzyme molecule can be used over and over again.
- Protein: Their structure is a complex, folded chain of amino acids. This 3D structure is absolutely critical to their function.
The specific region on an enzyme where the reaction occurs is called the active site. This is a small pocket or cleft with a unique shape, determined by the folding of the protein chain.
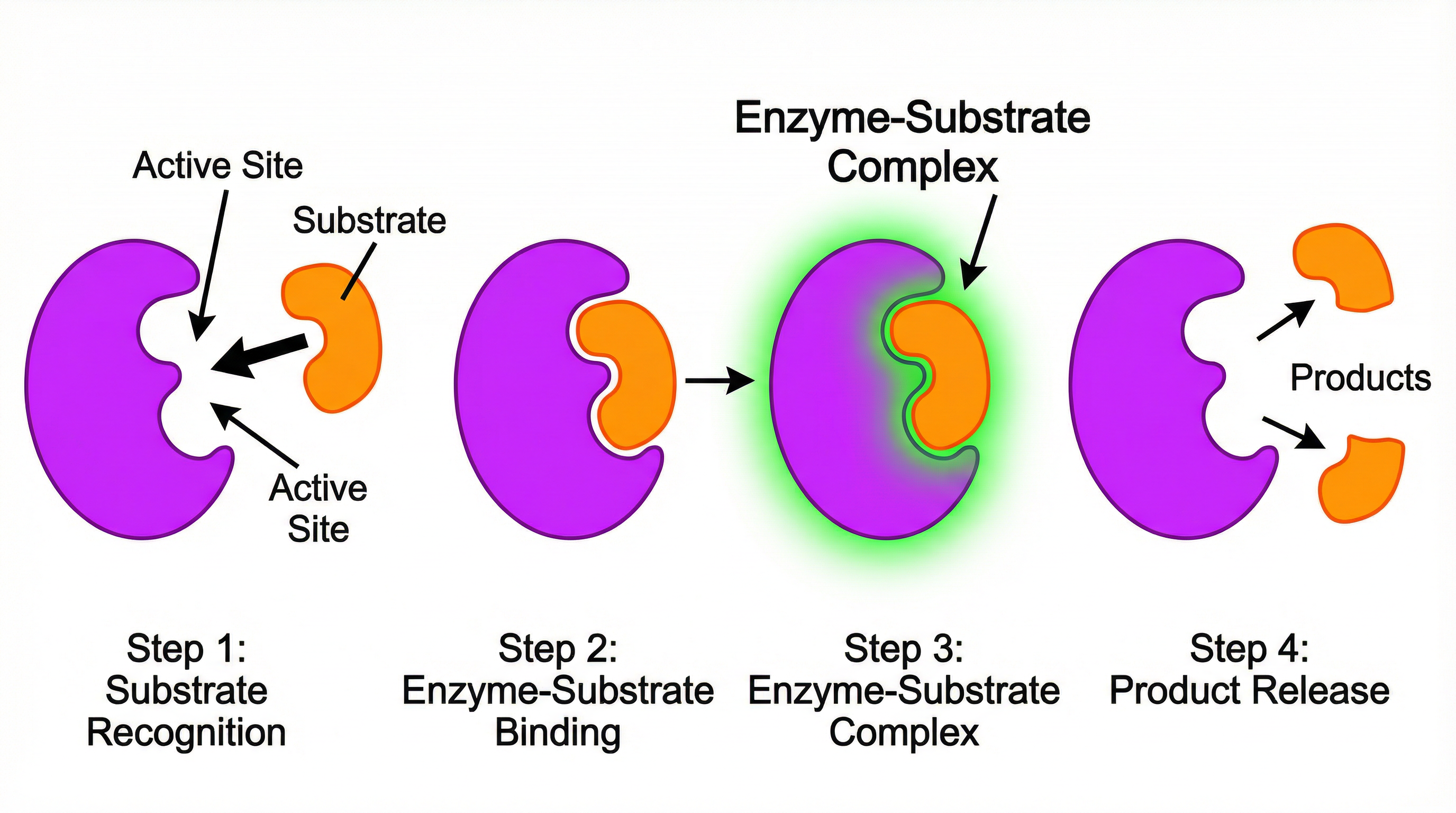
Concept 2: The Lock and Key Hypothesis
The lock and key hypothesis is a model used to explain the specificity of enzymes. It’s a concept that demands precise language in an exam.
- Specificity: Each type of enzyme will only act on one type of molecule, called its substrate.
- Complementary Shapes: The substrate molecule has a specific shape that is complementary to the shape of the active site of its corresponding enzyme. It is crucial to use the word ‘complementary’ and not ‘the same as’. Think of a key (the substrate) fitting perfectly into a lock (the enzyme’s active site).
- Enzyme-Substrate Complex: The substrate binds to the active site, forming an enzyme-substrate complex. This is where the chemical reaction takes place.
- Product Release: Once the reaction is complete, the resulting molecules, now called products, are released from the active site. The enzyme is unchanged and ready to bind with another substrate molecule.
Concept 3: Factors Affecting Enzyme Activity - Temperature
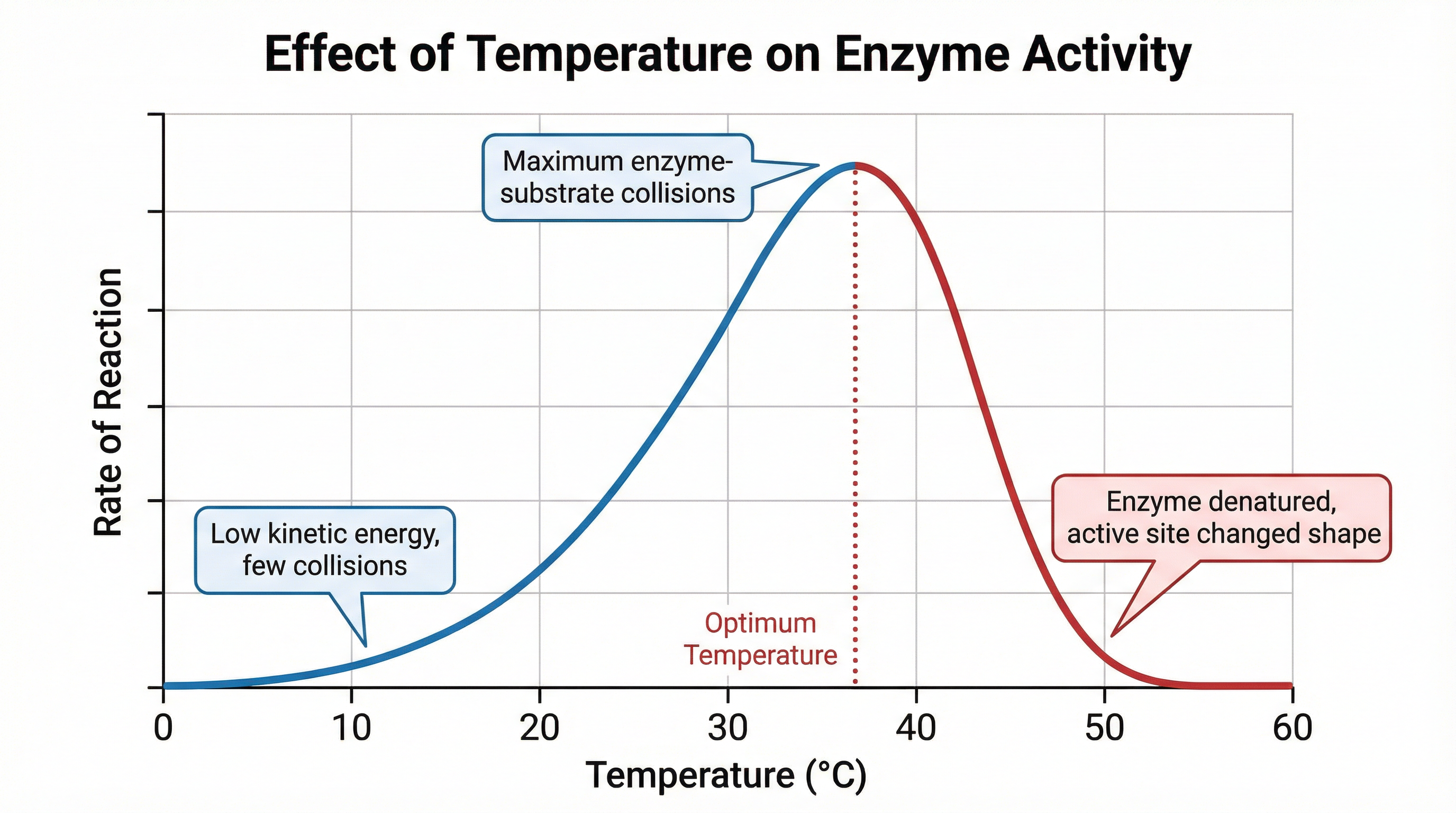
The rate at which an enzyme works is affected by several factors, with temperature being a key one that is frequently tested with graphs.
- Low Temperatures: At low temperatures, enzyme and substrate molecules have low kinetic energy. They move slowly, resulting in infrequent collisions. Therefore, the rate of reaction is low.
- Increasing Temperatures: As temperature rises, kinetic energy increases. Molecules move faster, leading to more frequent and energetic collisions between the active site and the substrate. This increases the rate of reaction.
- Optimum Temperature: This is the temperature at which the enzyme exhibits maximum activity. For most human enzymes, this is around 37°C. At this point, there is the highest frequency of successful collisions.
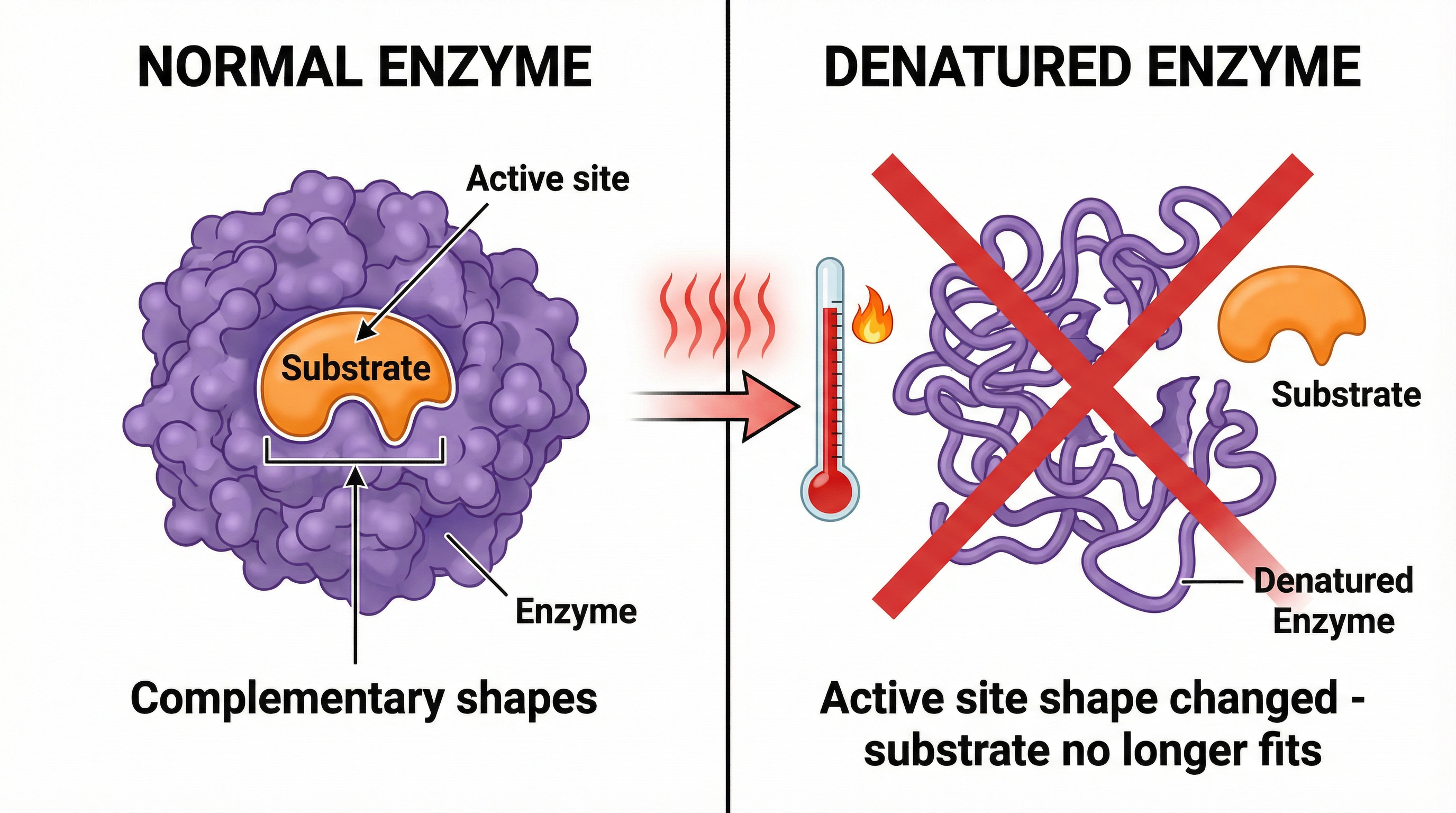
- High Temperatures: Beyond the optimum, the rate of reaction rapidly decreases. The high temperature provides too much kinetic energy, causing the enzyme to vibrate vigorously. This breaks the weak bonds that maintain the specific 3D shape of the protein. The active site changes shape permanently. This process is called denaturation. The substrate can no longer fit into the denatured active site, so the reaction stops.
It is a common mistake to say the enzyme is ‘killed’. Enzymes are not living things; the correct term is denatured.
Concept 4: Factors Affecting Enzyme Activity - pH
Like temperature, pH also has a significant effect on enzyme activity.
- Optimum pH: Every enzyme has an optimum pH at which its activity is highest. For example, pepsin, an enzyme in the stomach, works best at an acidic pH of 2, whereas trypsin in the small intestine works best at an alkaline pH of 8.
- Changes from the Optimum: If the pH moves too far above or below the optimum, the forces holding the protein’s 3D structure together are disrupted. This alters the shape of the active site, causing the enzyme to denature. The substrate can no longer bind, and the rate of reaction falls to zero.
Mathematical/Scientific Relationships
In enzyme experiments, you often measure the time it takes for a reaction to complete. To earn marks, you must be able to convert this into a rate.
- Formula: Rate of reaction = 1 / time
- Units: If time is measured in seconds (s), the rate is in ‘per second’ (s⁻¹).
- Example: If a reaction takes 50 seconds to complete, the rate is 1 / 50 = 0.02 s⁻¹.
- Alternative Formula: Sometimes, to avoid small decimals, the rate is calculated as: Rate = 1000 / time. In this case, the units are arbitrary and might be given as ‘units of rate’. Always check the question for the required format.
Practical Applications
Enzymes are not just abstract concepts; they are used widely in industry and are central to the required practical on investigating enzyme activity.
Industrial Uses
- Biological Washing Powders: Contain proteases and lipases to break down protein and fat stains (like blood and grease) at lower temperatures, saving energy.
- Baby Food Production: Proteases are used to pre-digest proteins, making the food easier for infants to digest.
- Lactose-Free Milk: The enzyme lactase is used to break down lactose (a sugar in milk) into simpler sugars (glucose and galactose) for people who are lactose intolerant.
Required Practical: Investigating the Effect of a Factor on Enzyme Activity
A typical experiment involves measuring the rate at which the enzyme amylase breaks down starch into maltose. The reaction can be timed by observing how long it takes for the starch to disappear, tested using iodine solution (which turns blue-black in the presence of starch).
- Apparatus: Test tubes, water bath, spotting tile, amylase solution, starch solution, iodine solution, stop clock, buffer solutions (for pH experiments).
- Method (for Temperature):
- Set up water baths at a range of temperatures (e.g., 10°C, 20°C, 30°C, 40°C, 50°C).
- Add a set volume of starch solution to a test tube in each water bath and allow it to reach the target temperature.
- Add a set volume of amylase solution to the starch, start the stop clock immediately.
- At regular intervals (e.g., every 30 seconds), take a sample from the mixture and add it to a drop of iodine on a spotting tile.
- Record the time taken for the iodine solution to remain brown/yellow, indicating all the starch has been broken down.
- Repeat the experiment at each temperature to ensure reliability.
- Control Variables: Enzyme concentration, substrate concentration, pH.
- Common Errors: Inaccurate temperature control, incorrect timing, cross-contamination of solutions.
{{asset:enzymes_podcast.wav