Study Notes
Overview
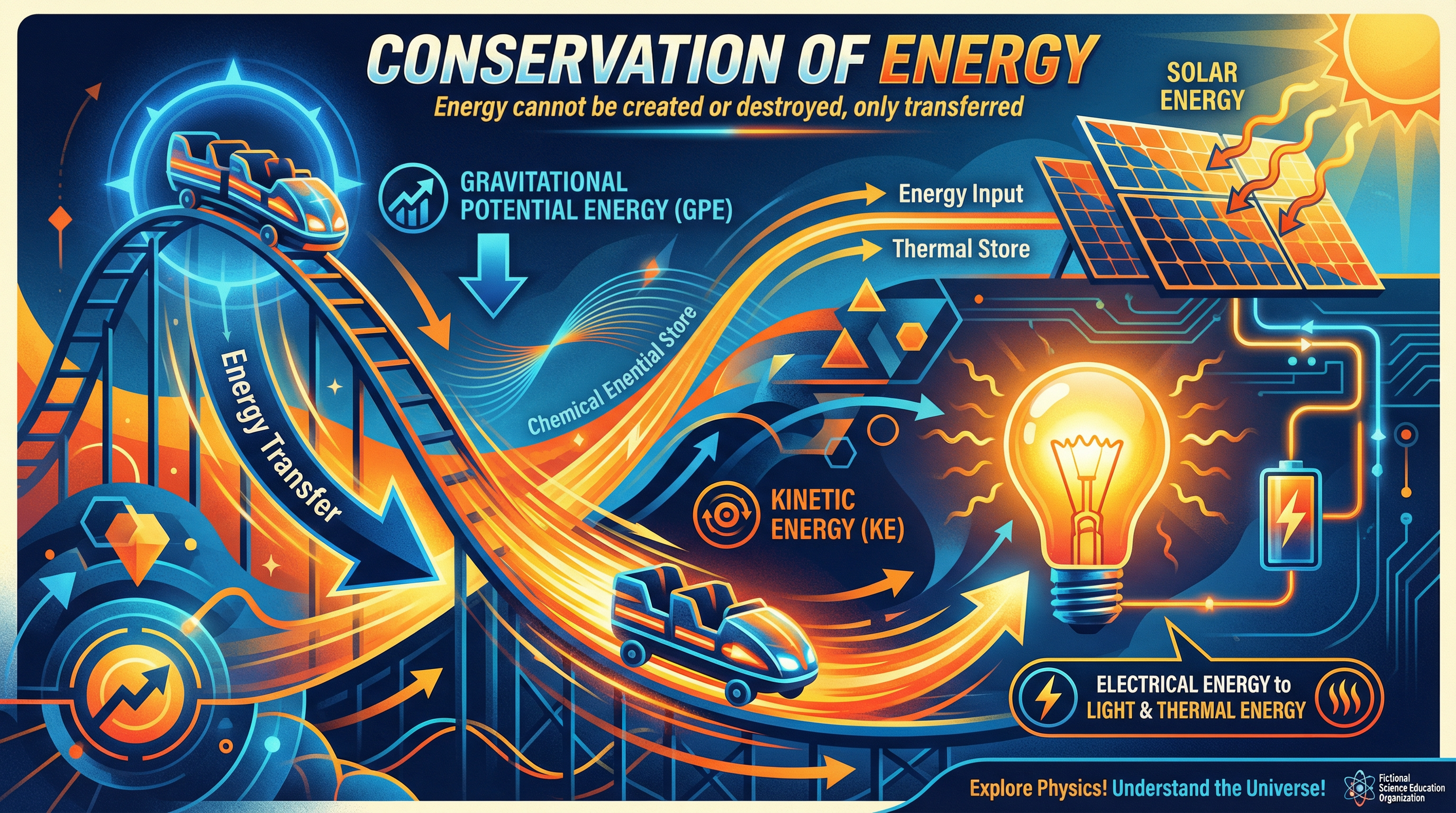
The principle of Conservation of Energy is one of the most fundamental and far-reaching concepts in all of physics. It states that energy cannot be created or destroyed, only transferred from one store to another, stored, or dissipated. For your Edexcel GCSE exam, this isn't just a phrase to memorise; it's a lens through which you must analyse everything from a falling apple to a power station. This topic, reference 1.4 in the specification, requires you to be precise with your language, confident with your calculations, and clear in your explanations. You will be expected to describe energy changes in closed systems, calculate kinetic and gravitational potential energy, and determine the efficiency of various devices. Examiners frequently link this topic to others like Forces (work done), Electricity (power ratings), and Waves (radiation), so a solid understanding here is crucial for picking up synoptic marks across the entire paper.
Key Concepts
Concept 1: Energy Stores and Transfers
In the world of Edexcel Physics, we don't talk about 'types' of energy. Instead, we talk about energy stores and energy transfers. Think of stores as bank accounts where energy is kept, and transfers as the methods of moving energy between those accounts. You must be ableto name the eight stores and four transfer pathways.
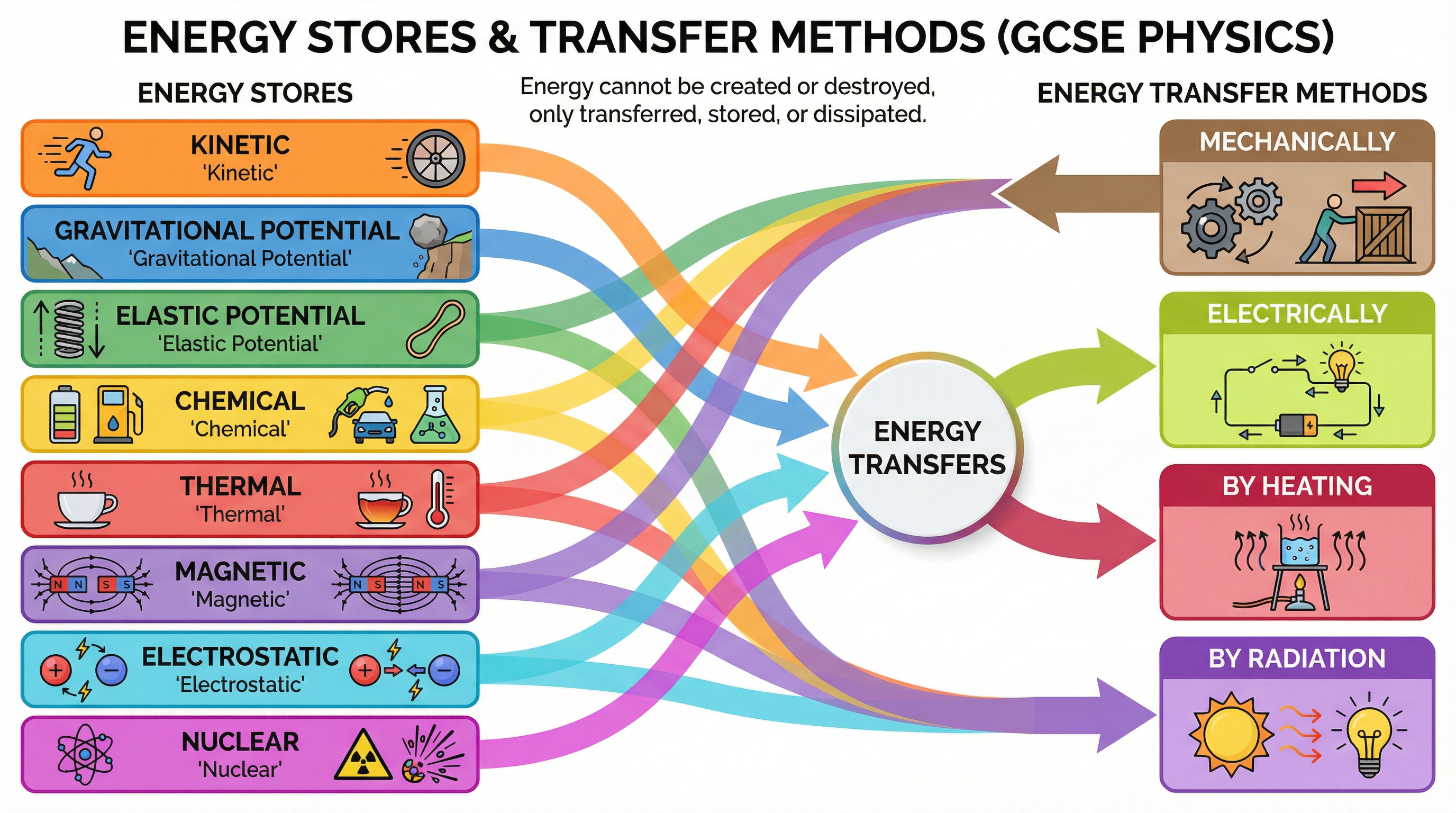
The Eight Energy Stores:
- Kinetic: The energy of a moving object.
- Gravitational Potential (GPE): Energy stored in an object due to its position in a gravitational field.
- Elastic Potential: Energy stored when an object is stretched or compressed.
- Chemical: Energy stored in the bonds between atoms (e.g., in food, fuel, batteries).
- Thermal: The total kinetic and potential energy of the particles in an object, often called heat.
- Magnetic: Energy stored when repelling poles have been pushed closer together or attracting poles have been pulled further apart.
- Electrostatic: Energy stored when repelling charges have been moved closer together or attracting charges have been pulled further apart.
- Nuclear: Energy stored in the nucleus of an atom.
The Four Energy Transfer Pathways:
- Mechanically: An object moving due to a force acting on it (e.g., pushing, pulling, stretching).
- Electrically: A charge moving through a potential difference (e.g., current in a circuit).
- By Heating: Energy transferred from a hotter object to a colder object.
- By Radiation: Energy transferred by waves (e.g., light, sound, infrared).
Example: When a ball is thrown upwards, energy is transferred mechanically from the kinetic store of the ball to its gravitational potential store. As it falls back down, energy is transferred from the gravitational potential store back to the kinetic store.
Concept 2: The Principle of Conservation of Energy and Closed Systems
The core principle is that the total energy of a closed system remains constant. A closed system is an isolated system where no energy can enter or leave. In reality, perfect closed systems are rare, but the concept is vital for calculations. When you analyse an energy transfer, the total energy input must equal the total energy output. However, not all of that output energy may be useful. Energy that is not usefully transferred is described as wasted. This wasted energy is dissipated, meaning it spreads out to the surroundings, increasing their thermal energy. This is why a light bulb gets hot – the wasted energy is dissipated as heat.
Crucial Exam Language: Do NOT say energy is 'lost' or 'used up'. The correct term is dissipated. Using this exact phrasing will earn you marks.
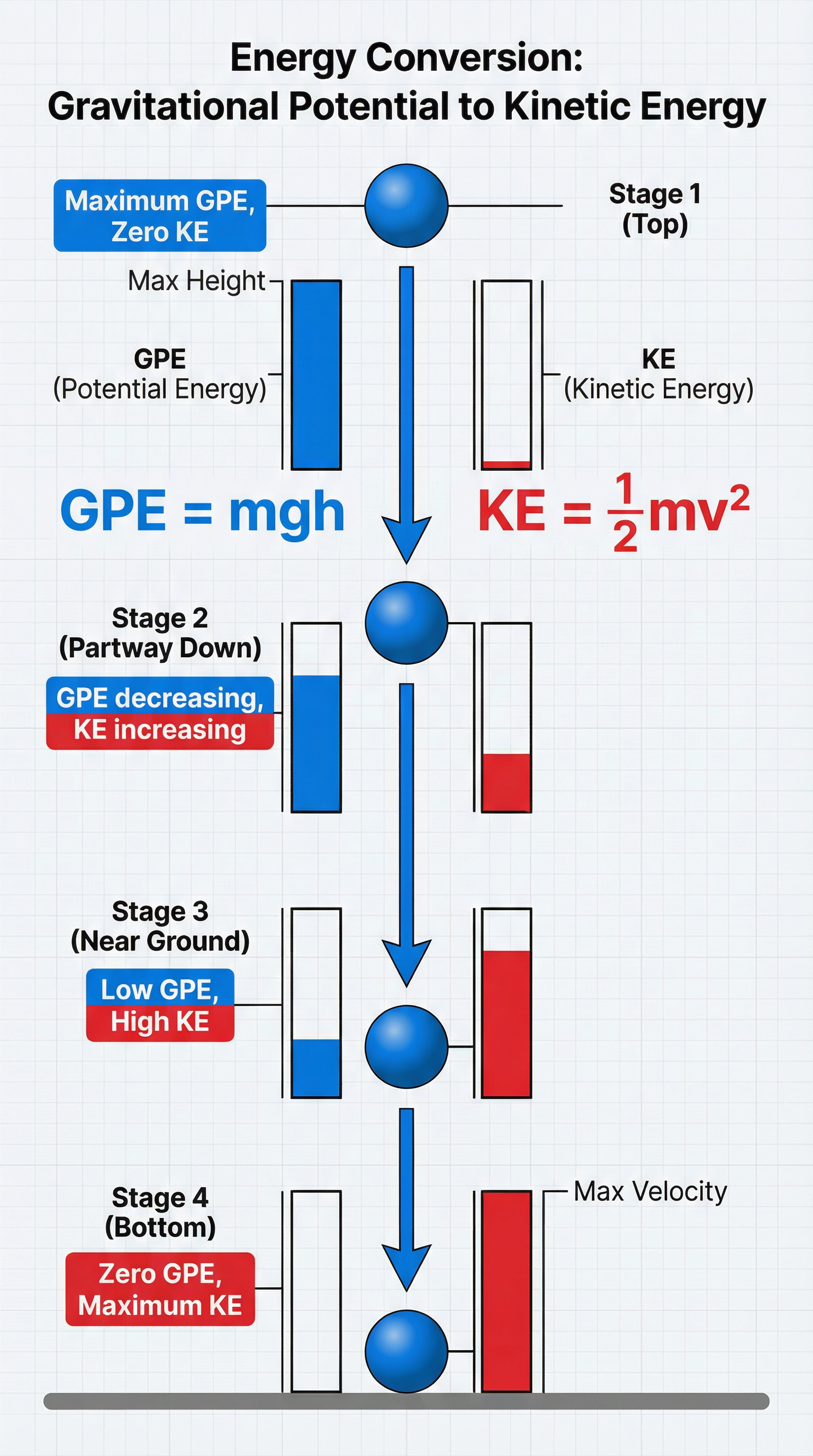
Concept 3: Mathematical Relationships (GPE and KE)
Two key calculations you must master are for Gravitational Potential Energy (GPE) and Kinetic Energy (KE).
**Gravitational Potential Energy (GPE)**This is the energy an object has because of its height.
GPE (J) = mass (kg) × gravitational field strength (N/kg) × height (m)
ΔEp = m × g × Δh
- ΔEp or GPE: Gravitational Potential Energy, in Joules (J).
- m: mass, in kilograms (kg).
- g: gravitational field strength, in Newtons per kilogram (N/kg). On Earth, this is approximately 9.8 N/kg, but in exams, you are often told to use 10 N/kg to simplify the maths.
- Δh: change in vertical height, in metres (m).
**Kinetic Energy (KE)**This is the energy of a moving object.
KE (J) = 1/2 × mass (kg) × (speed)² (m/s)²
Ek = 1/2 × m × v²
- Ek or KE: Kinetic Energy, in Joules (J).
- m: mass, in kilograms (kg).
- v: speed (or velocity), in metres per second (m/s).
Higher Tier Link: In a system where there is no air resistance or friction (like an object falling in a vacuum), the GPE lost is equal to the KE gained. So, mgh = 1/2 mv². This allows you to calculate the speed of a falling object without knowing the time it took to fall.
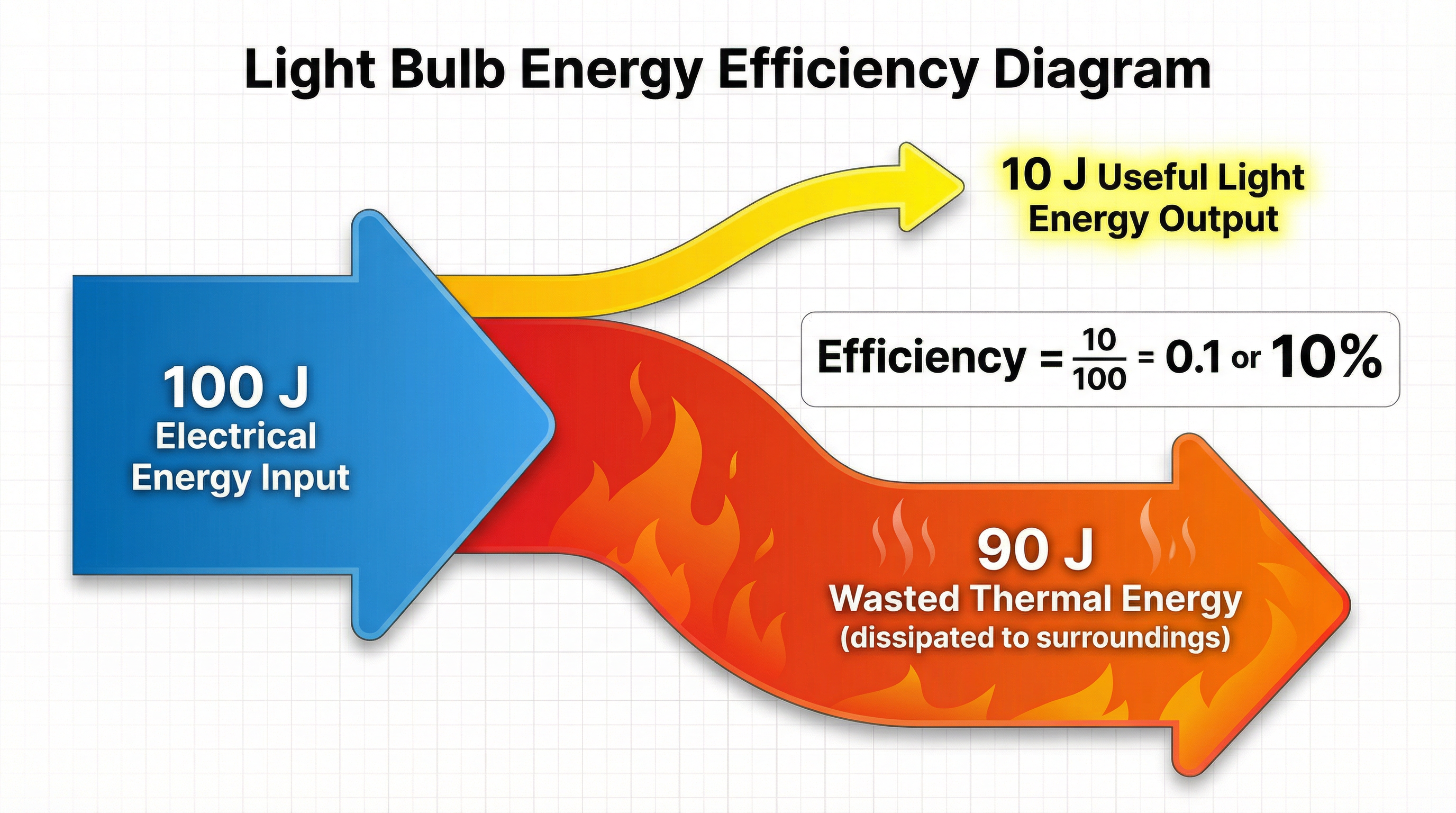
Concept 4: Efficiency
Efficiency is a measure of how good a device is at transferring energy into a useful form. No device is 100% efficient; some energy is always wasted (dissipated).
Efficiency = Useful energy output (J) / Total energy input (J)
Efficiency = Useful power output (W) / Total power input (W)
Efficiency is a ratio and has no units. It can be given as a decimal (e.g., 0.7) or a percentage (e.g., 70%).
Important: Efficiency can NEVER be greater than 1 (or 100%). If you calculate a value higher than 1, you have divided the numbers the wrong way around. This is a common mistake, so always do a quick sense-check of your answer.
Practical Applications
Reducing Unwanted Energy Transfers: In many systems, we want to reduce wasted energy to improve efficiency and save money. The two main methods are:
- Lubrication: Used in engines and machinery to reduce friction between moving parts. This reduces the amount of energy dissipated as heat.
- Thermal Insulation: Used in houses, flasks, and freezers. Materials with low thermal conductivity (insulators) are used to reduce the rate of energy transfer by heating. This keeps hot things hot and cold things cold.
Required Practical: Investigating Thermal Insulators
- Apparatus: Beakers, kettle, thermometer, stopwatch, insulating materials (e.g., bubble wrap, foil, wool), cardboard lid.
- Method: Wrap each beaker in a different insulating material. Fill each with the same volume of hot water at the same initial temperature. Place a lid on top to reduce evaporation. Record the temperature of the water in each beaker every 2 minutes for 20 minutes. The beaker that cools the slowest has the best insulator.
- Common Errors: Not using a lid, using different volumes of water, starting at different temperatures. Examiners test this by asking you to identify variables (independent, dependent, control) and evaluate the method.
Unit Conversions
- Energy: 1 kilojoule (kJ) = 1,000 Joules (J); 1 megajoule (MJ) = 1,000,000 Joules (J).
- Power: 1 kilowatt (kW) = 1,000 Watts (W); 1 megawatt (MW) = 1,000,000 Watts (W).
- Mass: 1 gram (g) = 0.001 kilograms (kg).
- Height/Distance: 1 centimetre (cm) = 0.01 metres (m); 1 kilometre (km) = 1,000 metres (m).
Candidates often lose marks for failing to convert to standard units (kg, m, s, J, W) before a calculation.
