Study Notes

Overview
Welcome to the essential guide for Edexcel GCSE Combined Science, Topic 2.2: Chemical Changes. This topic is a cornerstone of your chemistry paper, focusing on the reactions of acids and bases, the formation of salts, and the powerful process of electrolysis. It’s a topic that beautifully marries theoretical knowledge with practical application, which is why examiners feature it so heavily. You can expect to see a wide range of questions, from simple definitions (AO1) to applying your knowledge to unfamiliar scenarios (AO2) and evaluating experimental methods (AO3). A solid understanding here will not only secure you marks on this topic but also provide foundational knowledge for other areas of chemistry, such as rates of reaction and energy changes.
Key Concepts
Concept 1: Acids, Alkalis and pH
At its core, this topic is about ions in solution. An acid is a substance that, when dissolved in water, produces hydrogen ions (H⁺). For example, hydrochloric acid (HCl) dissociates in water to form H⁺ and Cl⁻ ions. An alkali is a soluble base that produces hydroxide ions (OH⁻) in aqueous solution. Sodium hydroxide (NaOH), for instance, dissolves to form Na⁺ and OH⁻ ions. It is critical to use the phrase ‘in aqueous solution’ as this is a key condition for these definitions that examiners look for.
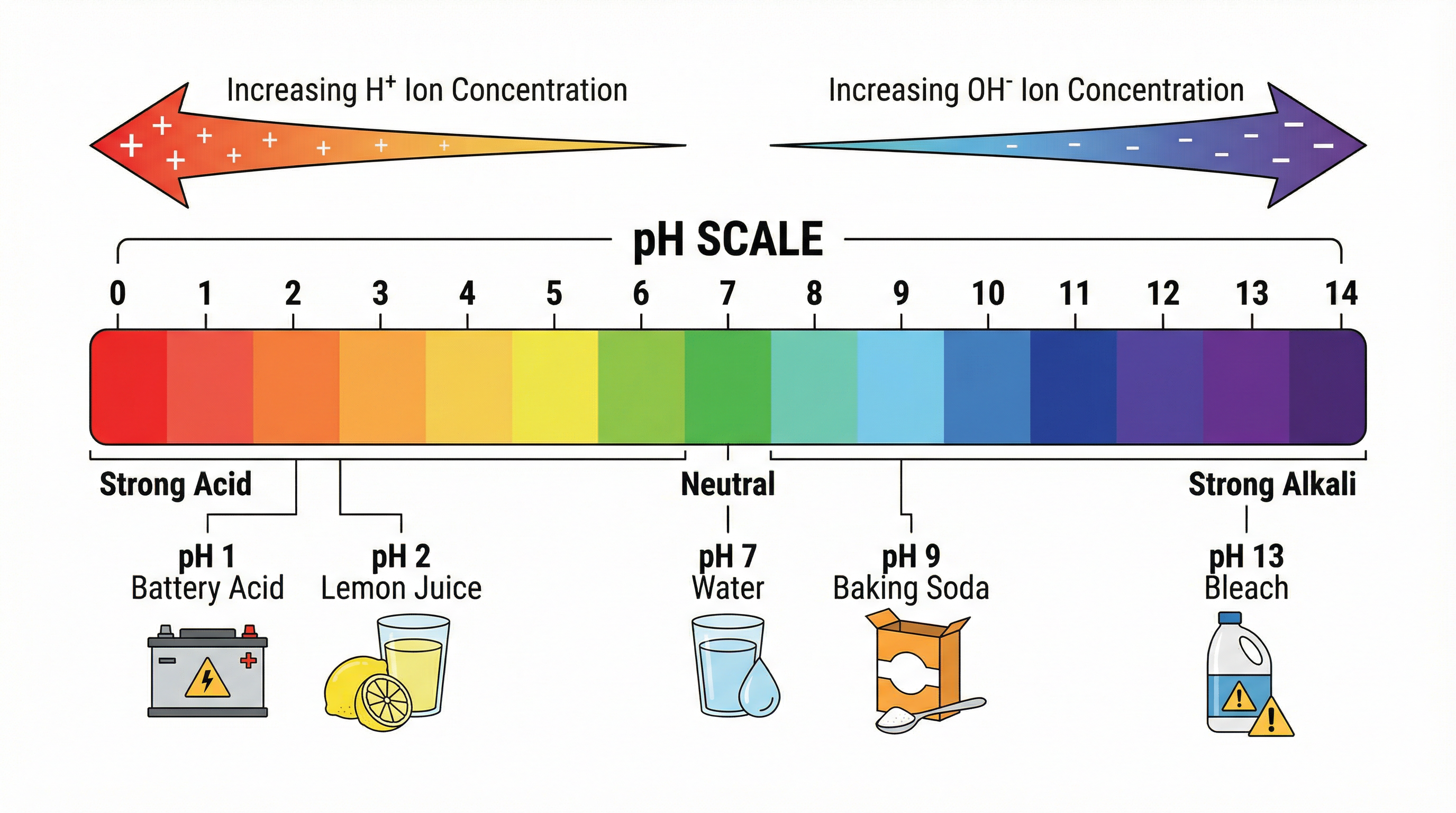
The pH scale is a measure of the concentration of H⁺ ions. It is a logarithmic scale from 0 to 14. A lower pH indicates a higher H⁺ concentration (more acidic), while a higher pH indicates a higher OH⁻ concentration (more alkaline). A pH of 7 is neutral, the point at which H⁺ and OH⁻ concentrations are equal. Because the scale is logarithmic, a change of one pH unit represents a tenfold change in H⁺ ion concentration. For example, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4.
Higher Tier Content: A crucial distinction is between strong and weak acids. A strong acid (e.g., HCl, H₂SO₄) fully ionises in water, meaning all its molecules release their H⁺ ions. A weak acid (e.g., ethanoic acid, CH₃COOH) only partially ionises, with most molecules remaining intact. This is different from concentrated vs dilute, which refers to the amount of acid dissolved per unit volume. You can have a dilute strong acid or a concentrated weak acid.
Concept 2: Neutralisation and Salt Preparation
Neutralisation is the reaction between an acid and a base. The fundamental reaction is the combination of a hydrogen ion and a hydroxide ion to form a water molecule. The ionic equation, which you must memorise, is:
**H⁺(aq) + OH⁻(aq) → H₂O(l)**Notice the state symbols – they are essential and often worth a mark. The other product of a neutralisation reaction is a salt. The specific salt formed depends on the acid and base used:
- Hydrochloric acid produces chloride salts.
- Sulfuric acid produces sulfate salts.
- Nitric acid produces nitrate salts.
Required Practical: Preparing a Soluble SaltThis is a common exam question. To prepare a pure, dry sample of a soluble salt from an insoluble base (like copper(II) oxide and sulfuric acid to make copper(II) sulfate), you must follow these steps:
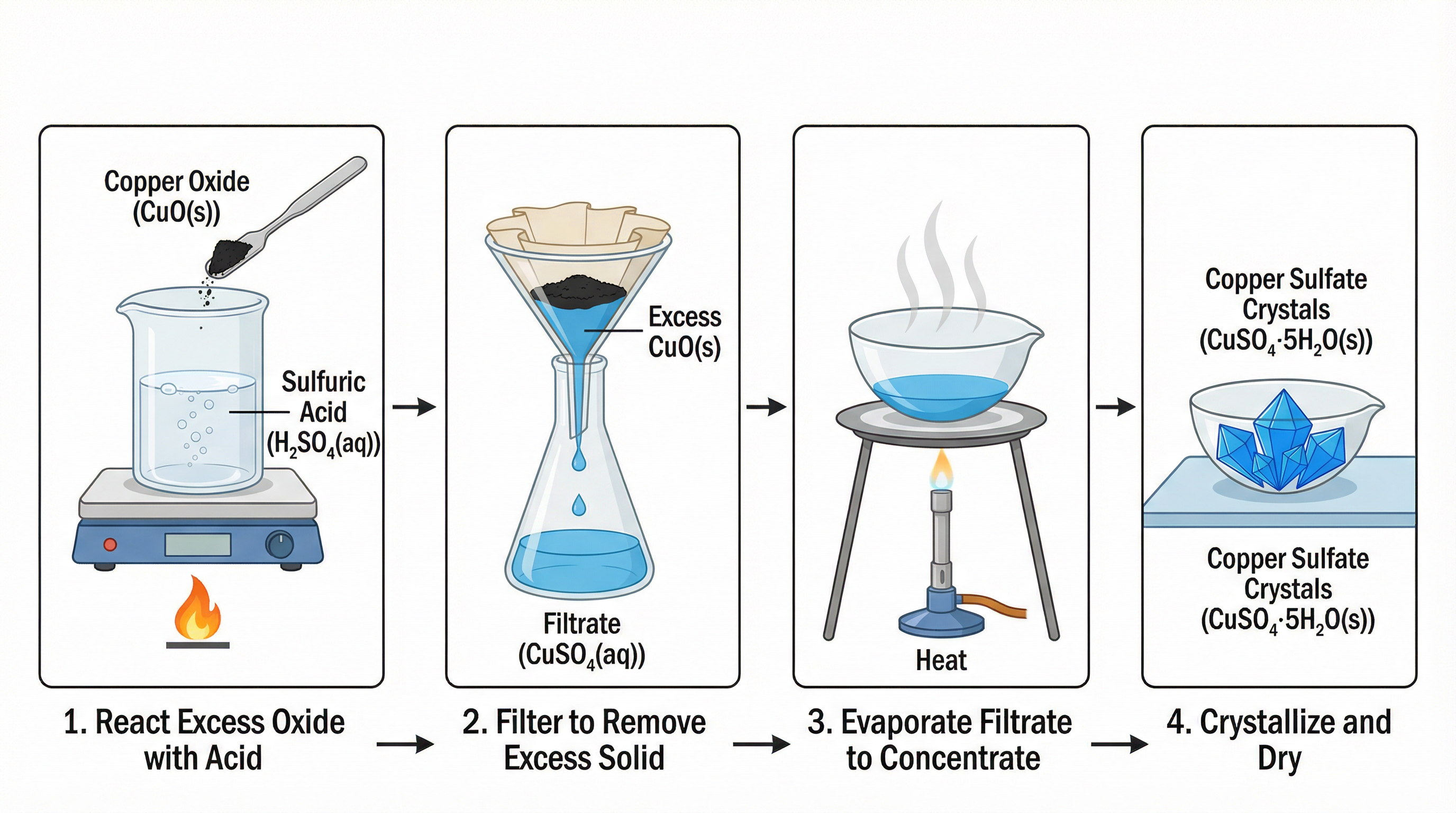
- React: Gently heat the acid and add the insoluble base in excess (i.e., until no more dissolves). This ensures all the acid is neutralised.
- Filter: Filter the mixture using filter paper and a funnel to remove the excess, unreacted base. The salt solution is the filtrate.
- Crystallise: Gently heat the filtrate in an evaporating dish to evaporate some of the water, increasing the concentration of the salt. Heat until the ‘point of crystallisation’ is reached (when crystals start to form on a glass rod dipped in the solution). Do not heat to dryness, as this can cause the salt to decompose.
- Dry: Leave the concentrated solution to cool slowly, allowing large crystals to form. Once formed, pat the crystals dry with filter paper.
Concept 3: Electrolysis
Electrolysis is the process of breaking down an ionic compound (the electrolyte) using electricity. It involves setting up an electrolytic cell with two electrodes: the anode (positive) and the cathode (negative).
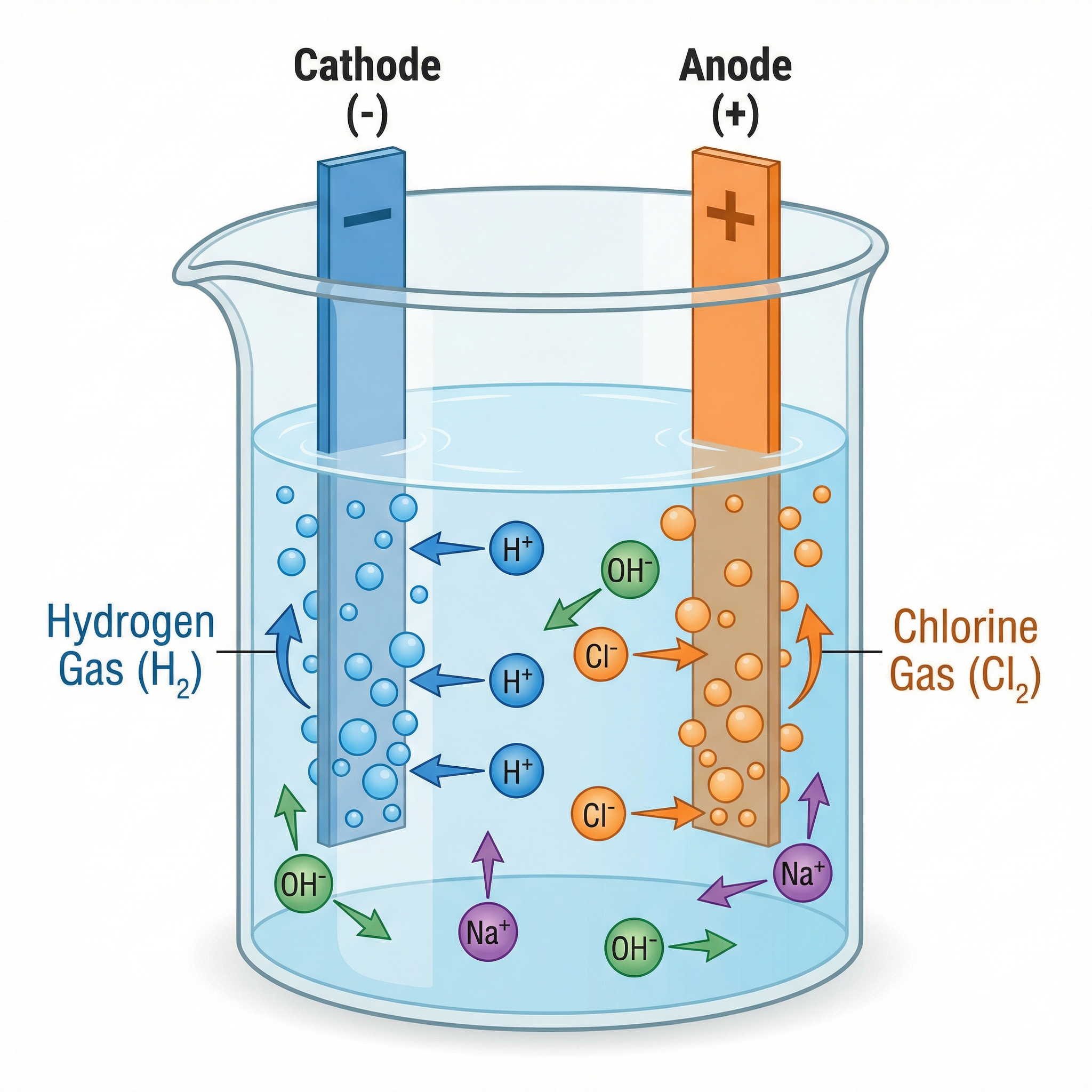
During electrolysis:
- Positively charged ions (cations) are attracted to the negative cathode, where they gain electrons (reduction).
- Negatively charged ions (anions) are attracted to the positive anode, where they lose electrons (oxidation).
A helpful mnemonic is PANIC: Positive Anode, Negative Is Cathode.
Electrolysis of Molten Ionic Compounds: This is the simpler case. For example, in molten lead(II) bromide (PbBr₂), the Pb²⁺ ions go to the cathode and form lead metal, while the Br⁻ ions go to theanode and form bromine gas.
- At the cathode: Pb²⁺ + 2e⁻ → Pb
- At the anode: 2Br⁻ → Br₂ + 2e⁻
Electrolysis of Aqueous Solutions: This is more complex because water itself can ionise to form H⁺ and OH⁻ ions, which compete with the ions from the electrolyte. The rules are:
- At the cathode (negative): If the metal is more reactive than hydrogen (e.g., sodium, potassium), hydrogen gas is produced. If the metal is less reactive than hydrogen (e.g., copper, silver), the metal is produced.
- At the anode (positive): If halide ions (Cl⁻, Br⁻, I⁻) are present, the corresponding halogen (chlorine, bromine, iodine) is produced. If no halide ions are present, oxygen gas is produced from the hydroxide ions.
Mathematical/Scientific Relationships
- Ionic Equation for Neutralisation: H⁺(aq) + OH⁻(aq) → H₂O(l) (Must memorise)
- Higher Tier Half-Equations: These describe the process of oxidation or reduction at each electrode. For example, in the electrolysis of aqueous sodium chloride:
- Cathode: 2H⁺(aq) + 2e⁻ → H₂(g) (Must memorise)
- Anode: 2Cl⁻(aq) → Cl₂(g) + 2e⁻ (Must memorise)
Practical Applications
- Neutralisation: Used in farming to treat acidic soil with lime (calcium oxide), and in treating indigestion with antacids (which are bases).
- Salt Preparation: Essential for creating fertilisers, food flavourings, and many industrial chemicals.
- Electrolysis: Used to extract reactive metals like aluminium from their ores, to produce chlorine and sodium hydroxide (a vital industrial chemical), and for electroplating objects with a thin layer of metal for protection or decoration.
