Study Notes
Overview
Planning investigations is a cornerstone of scientific practice and a major focus in the Edexcel GCSE Combined Science specification. This topic, falling under section 4.1, is primarily assessed through AO3 (Assessment Objective 3), which accounts for a substantial 60% of the marks. This means your ability to devise, critique, and refine experimental methods is heavily tested. Questions will challenge you to write a clear, logical plan for a given scenario, often in an unfamiliar context. Mastering this skill involves understanding variables, selecting appropriate apparatus, ensuring reliability and validity, and structuring your response in a way that examiners can easily credit. This guide will equip you with the techniques and knowledge to confidently tackle these high-stakes questions and turn them into a reliable source of marks.
Key Concepts
Concept 1: Variables
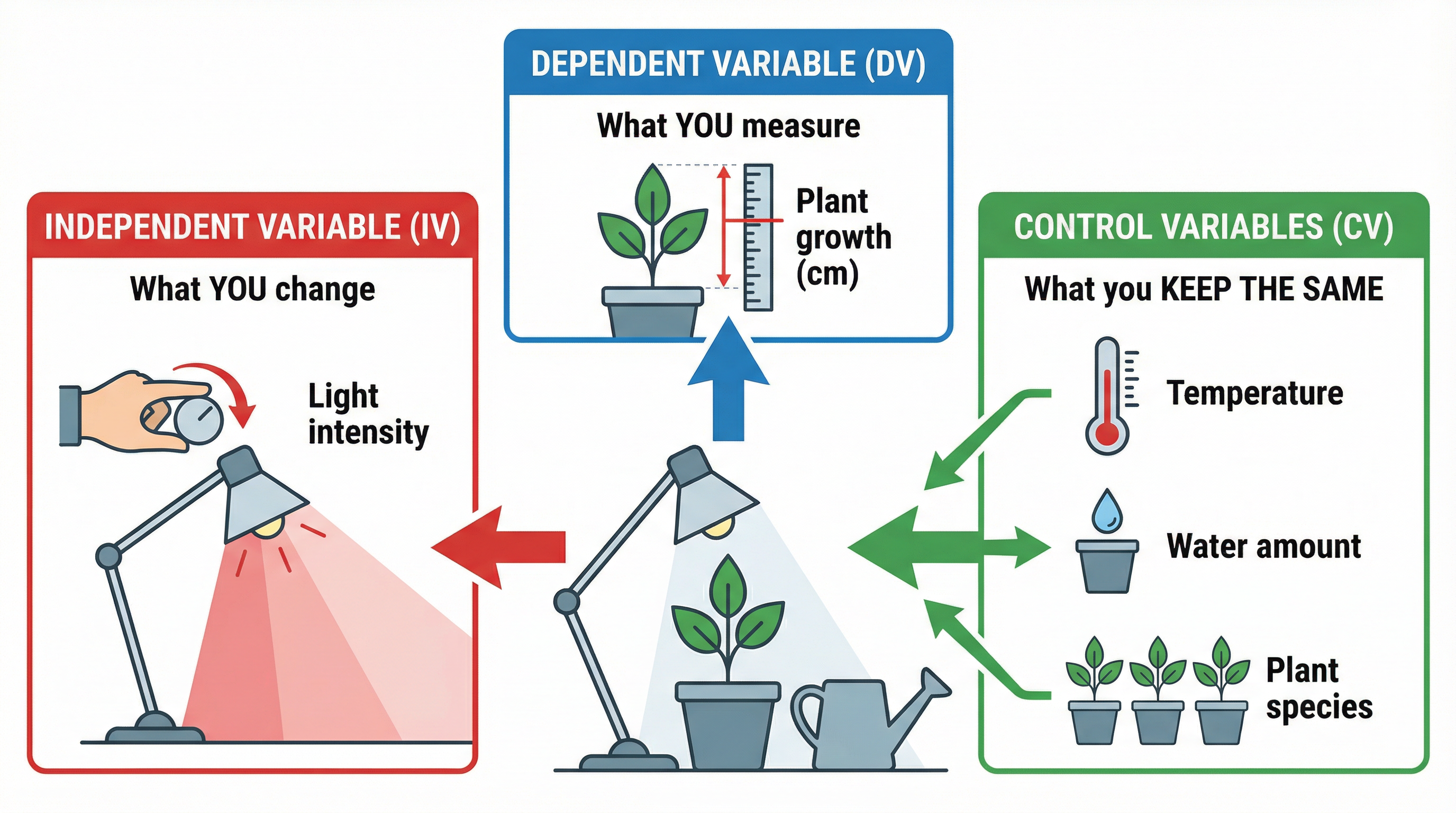
At the heart of any experiment are variables – factors that can be changed, measured, or controlled. Getting these right is fundamental to creating a valid investigation. There are three types:
- Independent Variable (IV): This is the single factor that you, the scientist, deliberately change or manipulate to see what effect it has. Think of it as the "cause". For example, in an experiment to see how light intensity affects the rate of photosynthesis, the light intensity is the IV.
- Dependent Variable (DV): This is the factor that you measure to see the effect of changing the IV. It "depends" on the independent variable. In the photosynthesis example, the DV could be the volume of oxygen produced per minute.
- Control Variables (CVs): These are all the other factors that could potentially affect the outcome of your experiment, so you must keep them constant to ensure a fair test. If they are not controlled, you cannot be certain that the changes in the DV are solely due to the changes in the IV. In the photosynthesis experiment, control variables would include temperature, carbon dioxide concentration, and the species of plant used.
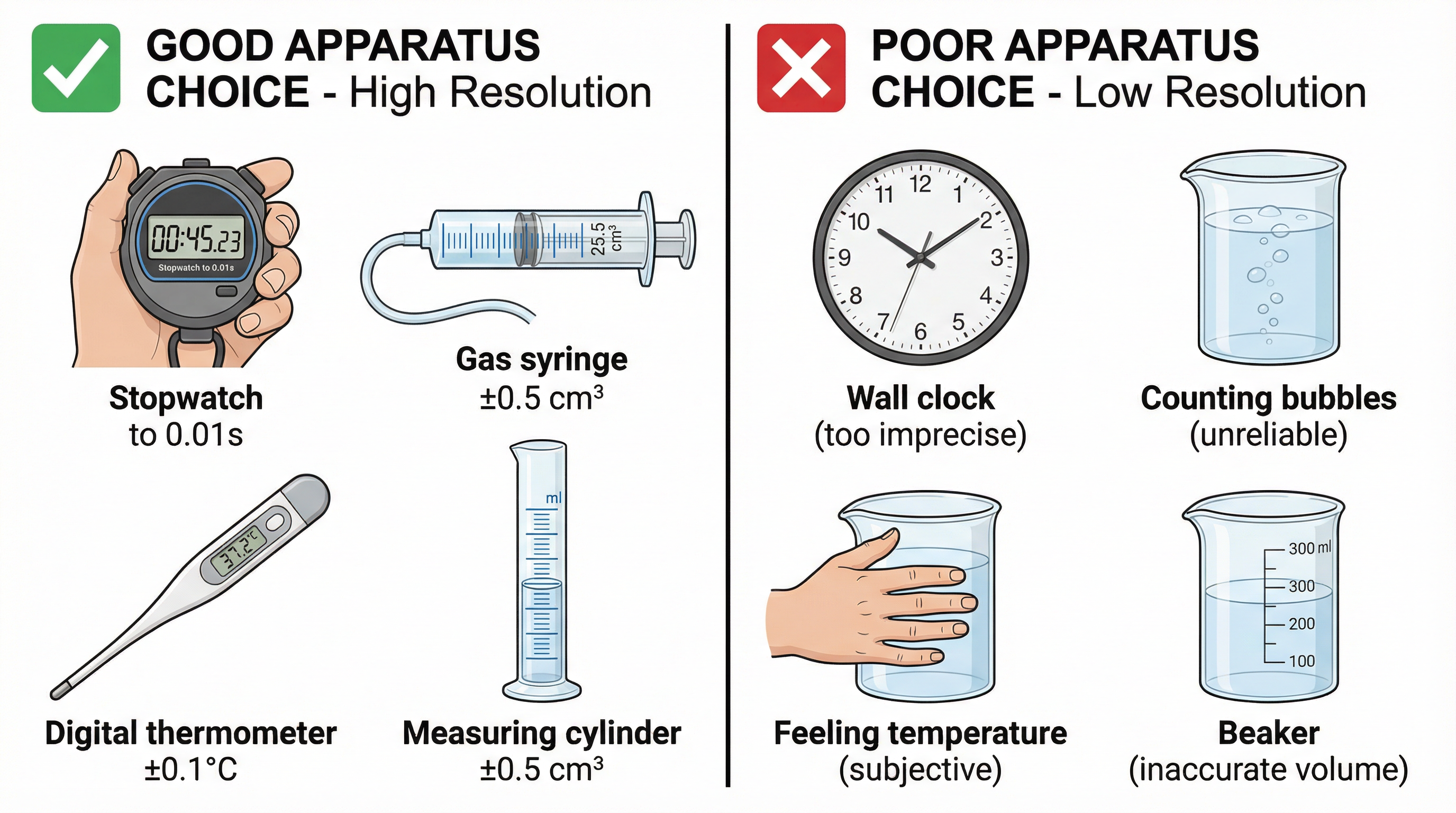
Concept 2: Apparatus and Resolution
Choosing the right tool for the job is critical for obtaining accurate and precise data. Examiners award marks for selecting apparatus with a suitable resolution for the measurement being taken. Resolution refers to the smallest change a measuring instrument can detect.
- High-Resolution Choices: For measuring time, a digital stopwatch (resolution: 0.01s) is far better than a wall clock. For volume, a measuring cylinder or gas syringe provides much greater precision than a beaker. A digital thermometer (resolution: 0.1°C) is superior to a simple lab thermometer.
- Justifying Your Choice: You should always be prepared to justify your apparatus choice. For example, "A gas syringe is used to measure the volume of gas produced as it has a resolution of ±0.5 cm³, which is more precise than counting bubbles, an unreliable and subjective method."
Concept 3: Reliability, Accuracy, and Validity
These three terms are often confused, but they mean different things:
- Reliability: This is about the consistency of your results. If you repeat the experiment, will you get similar outcomes? To ensure reliability, you must repeat your measurements at least three times and then calculate a mean. This allows you to spot and discard anomalous results.
- Accuracy: This is how close your measurement is to the true value. Using high-resolution, correctly calibrated apparatus improves accuracy.
- Validity: This refers to whether your experiment is a fair test that actually investigates what you set out to investigate. A valid experiment has only one independent variable, and all other potential variables are controlled. If you fail to control a key variable, your test is invalid because you can't be sure what caused the result.
Mathematical/Scientific Relationships
Calculating the Mean
When you have repeated your measurements, you need to calculate the mean (average) result. This is a fundamental skill.
Formula:
Mean = Sum of all measurements / Number of measurements
Example: A student measures the time taken for a reaction at 30°C three times and gets the results: 25.2s, 25.5s, and 25.1s.
Mean = (25.2 + 25.5 + 25.1) / 3 = 75.8 / 3 = 25.27s
Important: When an anomalous result is identified (one that does not fit the pattern), it should be excluded from the calculation of the mean.
Practical Applications
This topic is directly tested through required practicals. For example, in investigations into the effect of pH on enzyme activity, you would be expected to plan how to vary the pH (the IV) using different buffer solutions, measure the rate of reaction (the DV) by timing how long it takes for a substrate to disappear, and control variables like temperature (using a water bath) and enzyme concentration.