Study Notes
Overview
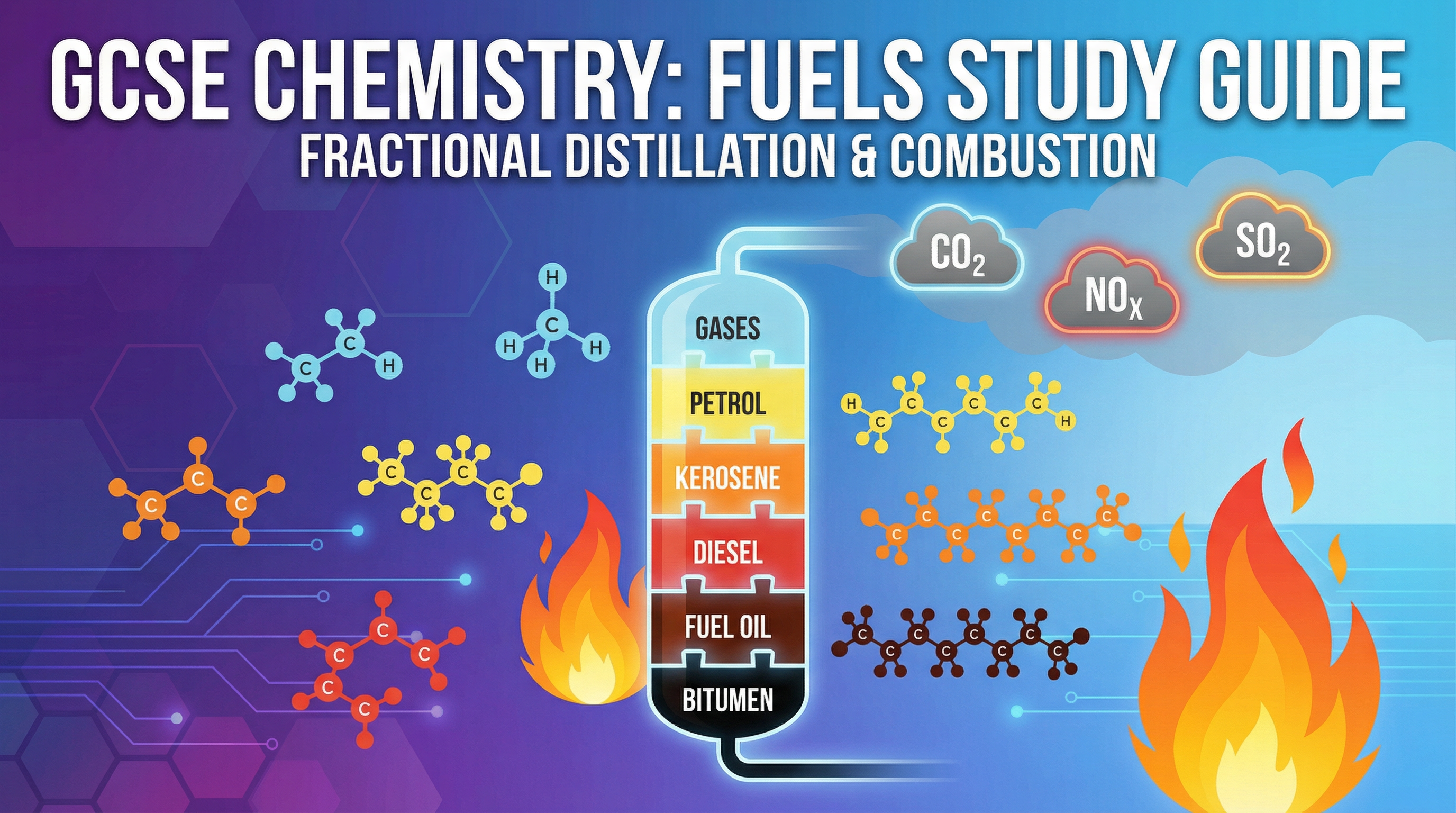
Welcome to your deep dive into Topic 2.6: Fuels. This topic is a cornerstone of your Edexcel GCSE Combined Science course, focusing on how we extract and use energy from one of the world's most vital resources: crude oil. You'll explore the industrial process of fractional distillation, linking the physical properties of hydrocarbons to their molecular structure. We'll also investigate the chemical reactions of combustion and critically evaluate the environmental consequences of burning fuels. Examiners frequently test this area through a mix of short-answer questions (AO1), application of knowledge (AO2), and longer evaluative tasks (AO3), so a solid understanding is crucial for achieving a high grade. This guide will equip you with the key concepts, exam techniques, and memory aids to tackle these questions with confidence.
Key Concepts
Concept 1: Crude Oil and Fractional Distillation
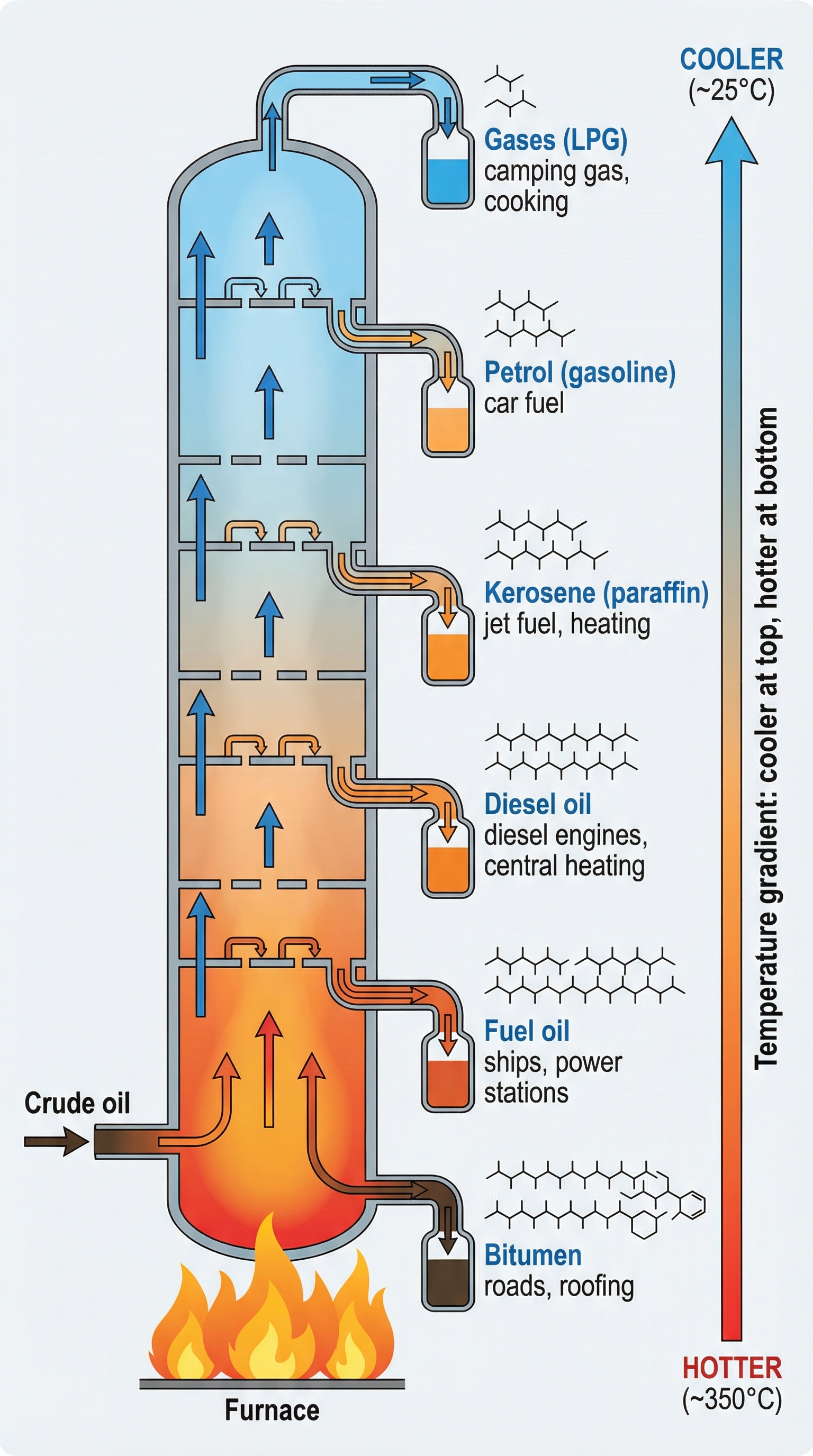
Crude oil is a complex mixture of hydrocarbons—compounds containing only carbon and hydrogen atoms. These hydrocarbons vary in the length of their carbon chains. To make crude oil useful, we must separate it into fractions, which are simpler mixtures with similar properties. This separation is achieved through fractional distillation, a physical process that exploits the differences in boiling points among the hydrocarbon chains.
The Process:
- Vaporisation: Crude oil is heated in a furnace to a high temperature (around 350°C), causing most of the hydrocarbons to turn into gas (vaporise).
- Entering the Column: The hot mixture of liquid and vapour is pumped into the bottom of a tall fractionating column.
- Temperature Gradient: The column has a distinct temperature gradient—it is very hot at the bottom and gradually gets cooler towards the top. This is the most critical point to remember for exam answers.
- Rising and Condensing: The hot hydrocarbon vapours rise up the column. As they rise, they cool down. When a vapour reaches a level in the column that is at or below its boiling point, it condenses back into a liquid and is collected on a tray.
- Separation by Chain Length:
- Long-chain hydrocarbons: These have strong intermolecular forces, requiring more energy to break. They have high boiling points, so they condense and are collected in the hotter, lower sections of the column (e.g., Fuel Oil, Bitumen).
- Short-chain hydrocarbons: These have weak intermolecular forces, requiring less energy to break. They have low boiling points, so they continue to rise to the cooler, higher sections of the column before condensing (e.g., Petrol, Gases).
Concept 2: Properties of Hydrocarbon Fractions
The length of the hydrocarbon chain dictates the physical properties of each fraction. Examiners expect you to be able to describe and explain these trends.
| Property | Trend as Chain Length Increases | Explanation (Why it works) | Example |
|---|---|---|---|
| Boiling Point | Increases | Larger molecules have stronger intermolecular forces of attraction, which require more energy (heat) to overcome. | Bitumen (long chains) has a very high boiling point. |
| Viscosity | Increases | Stronger intermolecular forces make it harder for the molecules to slide past each other, so the liquid is thicker. | Treacle (high viscosity) vs. water (low viscosity). |
| Flammability | Decreases | Shorter chains are more volatile (turn into vapour easily) and ignite more readily. | Petrol (short chains) is highly flammable. |
Concept 3: Combustion of Fuels
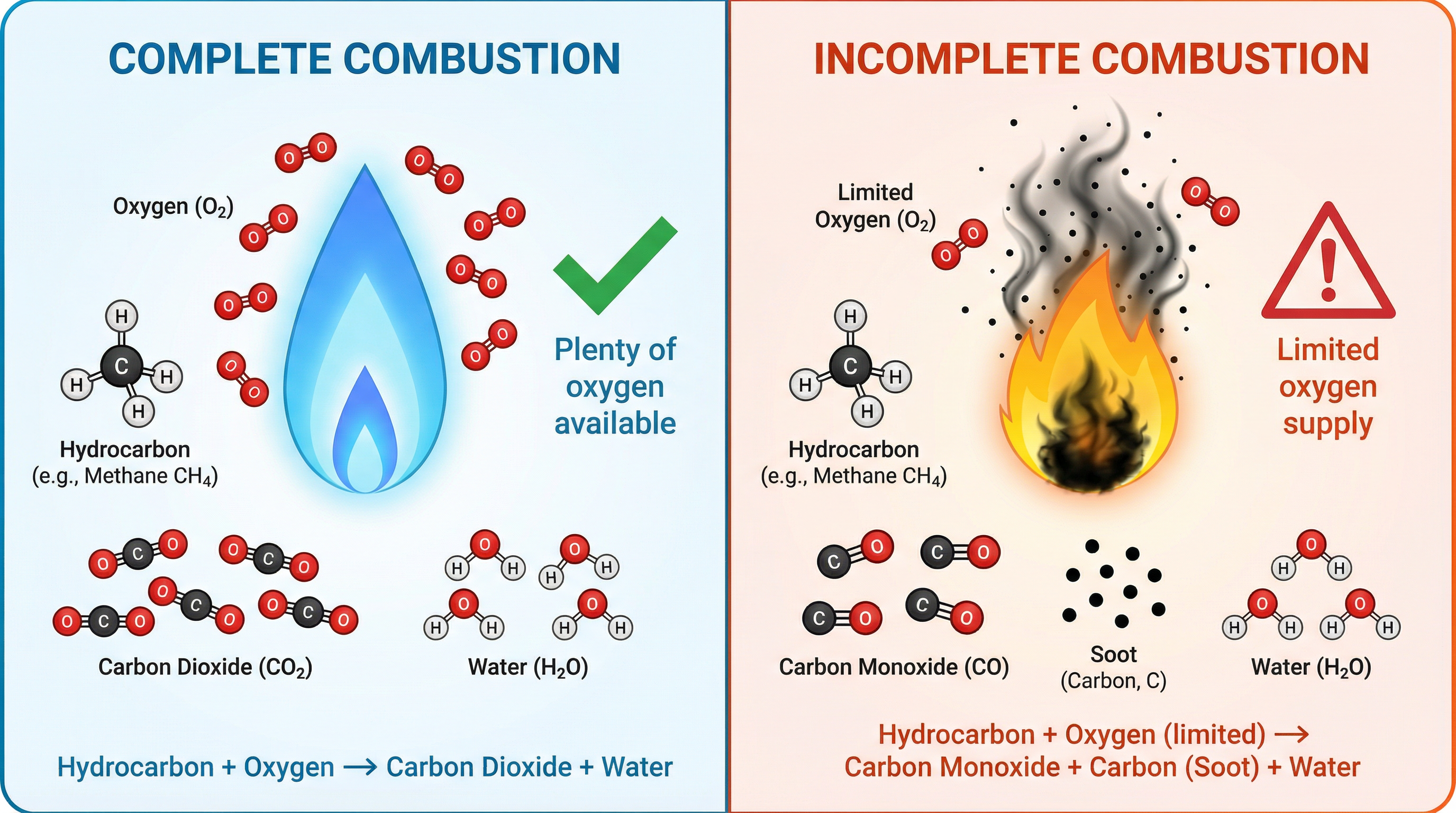
Combustion is the scientific term for burning. It is an exothermic reaction where a fuel reacts with oxygen to release energy. There are two types of combustion you must know.
- Complete Combustion: Occurs when there is a plentiful supply of oxygen. It releases the maximum amount of energy. The only products are carbon dioxide (CO2) and water (H2O).
- General Equation: Hydrocarbon + Oxygen → Carbon Dioxide + Water
- Incomplete Combustion: Occurs when the supply of oxygen is limited. It releases less energy. The products can include carbon monoxide (CO), carbon (C) (seen as soot), and water (H2O).
- General Equation: Hydrocarbon + Oxygen (limited) → Carbon Monoxide + Carbon + Water
Concept 4: Environmental Impact of Combustion
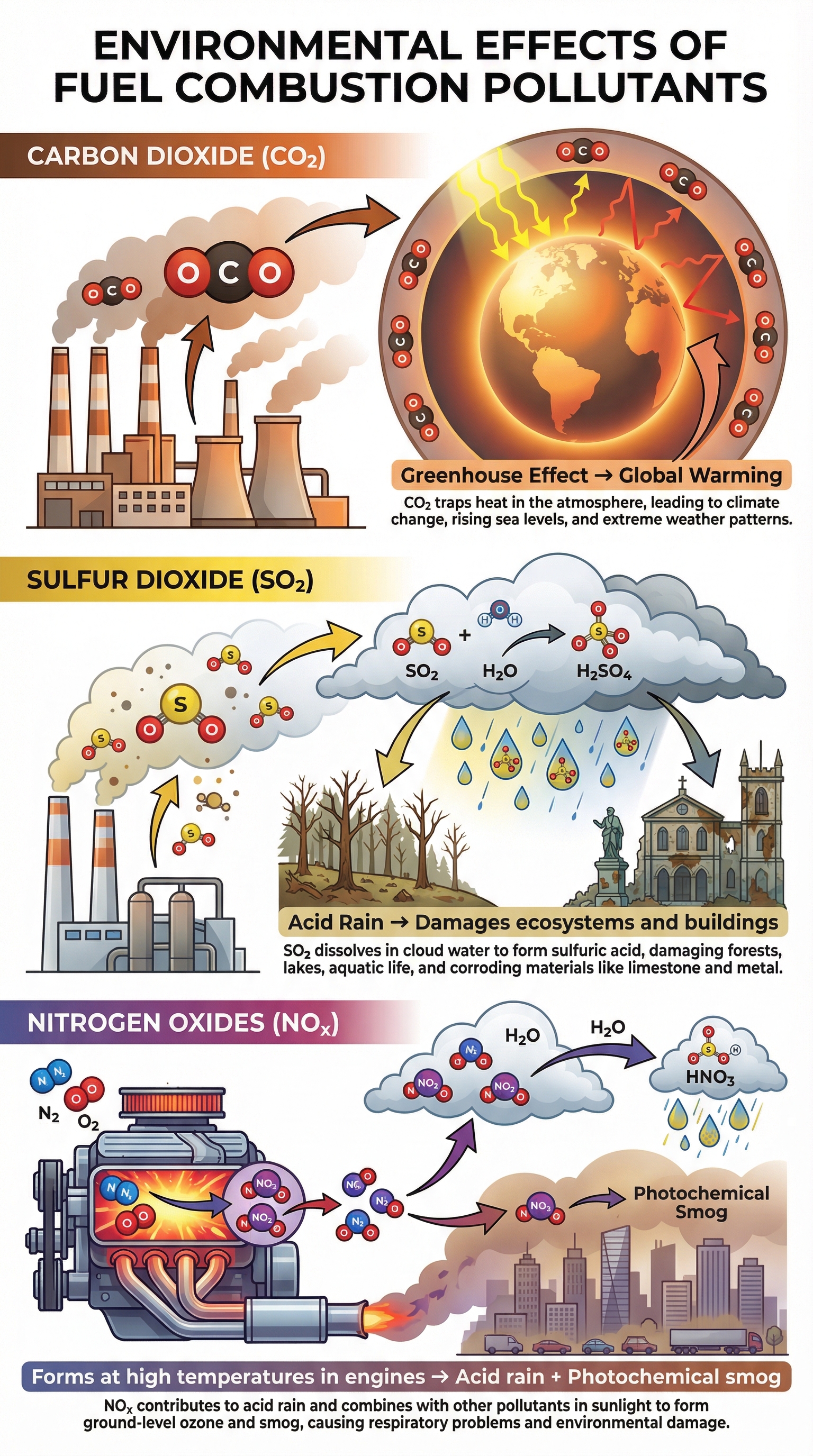
Burning fuels releases several pollutants into the atmosphere, each with significant environmental consequences. This is a common topic for 6-mark evaluation questions.
- Carbon Dioxide (CO2): A greenhouse gas. It absorbs infrared radiation emitted from the Earth's surface, trapping heat in the atmosphere. This enhanced greenhouse effect leads to global warming and climate change.
- Sulfur Dioxide (SO2): Produced when sulfur impurities (present in some fossil fuels) burn in oxygen. SO2 dissolves in water droplets in clouds to form sulfuric acid, which then falls as acid rain. Acid rain damages limestone buildings, harms aquatic ecosystems by lowering the pH of lakes, and kills trees.
- Nitrogen Oxides (NOx): Formed under the high-pressure and high-temperature conditions inside internal combustion engines (e.g., in cars). Nitrogen and oxygen from the air (not the fuel) are forced to react. Nitrogen oxides contribute to acid rain and photochemical smog.
Mathematical/Scientific Relationships
While this topic is less calculation-heavy, you must be proficient with chemical equations.
**1. Complete Combustion of Methane (Must memorise)**CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l)
This shows one molecule of methane reacts with two molecules of oxygen to produce one molecule of carbon dioxide and two molecules of water.
2. Incomplete Combustion of Methane (Must memorise)
2CH₄(g) + 3O₂(g) → 2CO(g) + 4H₂O(l)
This is one possible equation; the key is showing CO as a product.
Practical Applications
- Required Practical: There is no specific required practical for this topic in Edexcel GCSE Combined Science. However, questions may test your understanding of experimental techniques related to properties of hydrocarbons, such as comparing the viscosity of different oils by timing how long they take to flow down a ramp.
- Cracking: A key industrial process linked to fuels. Long-chain hydrocarbons from fractional distillation (which are in low demand) are broken down into more useful short-chain hydrocarbons (like petrol, which is in high demand) and alkenes. This process requires high temperatures (600-700°C) and a catalyst (silica or alumina).