Study Notes
Overview
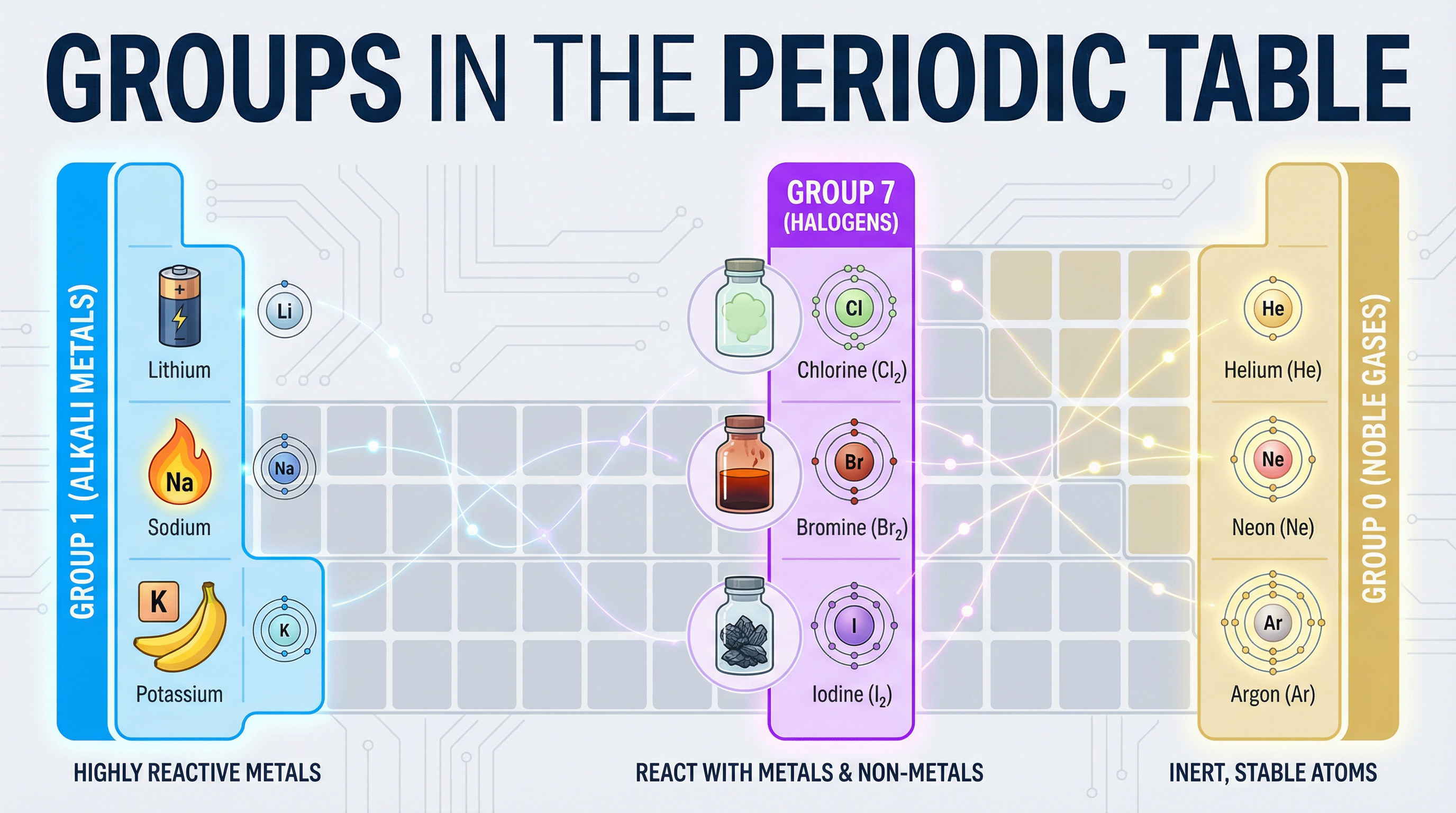
Welcome to your deep dive into Topic 2.4: Groups in the Periodic Table. This topic is fundamental to understanding how an element's position in the periodic table dictates its chemical properties. We will focus on three key groups: the highly reactive Group 1 alkali metals, the reactive Group 7 halogens, and the inert Group 0 noble gases. A significant portion of marks in the exam are awarded for explaining the trends in reactivity down these groups, particularly for Higher Tier candidates who must use concepts like atomic radius and electron shielding. You will also be tested on your knowledge of the physical properties of these elements and their reactions. This topic frequently appears in structured questions requiring both recall (AO1) and application of knowledge (AO2), so a solid grasp of the core principles is essential for success.
Key Concepts
Concept 1: Group 1 – The Alkali Metals
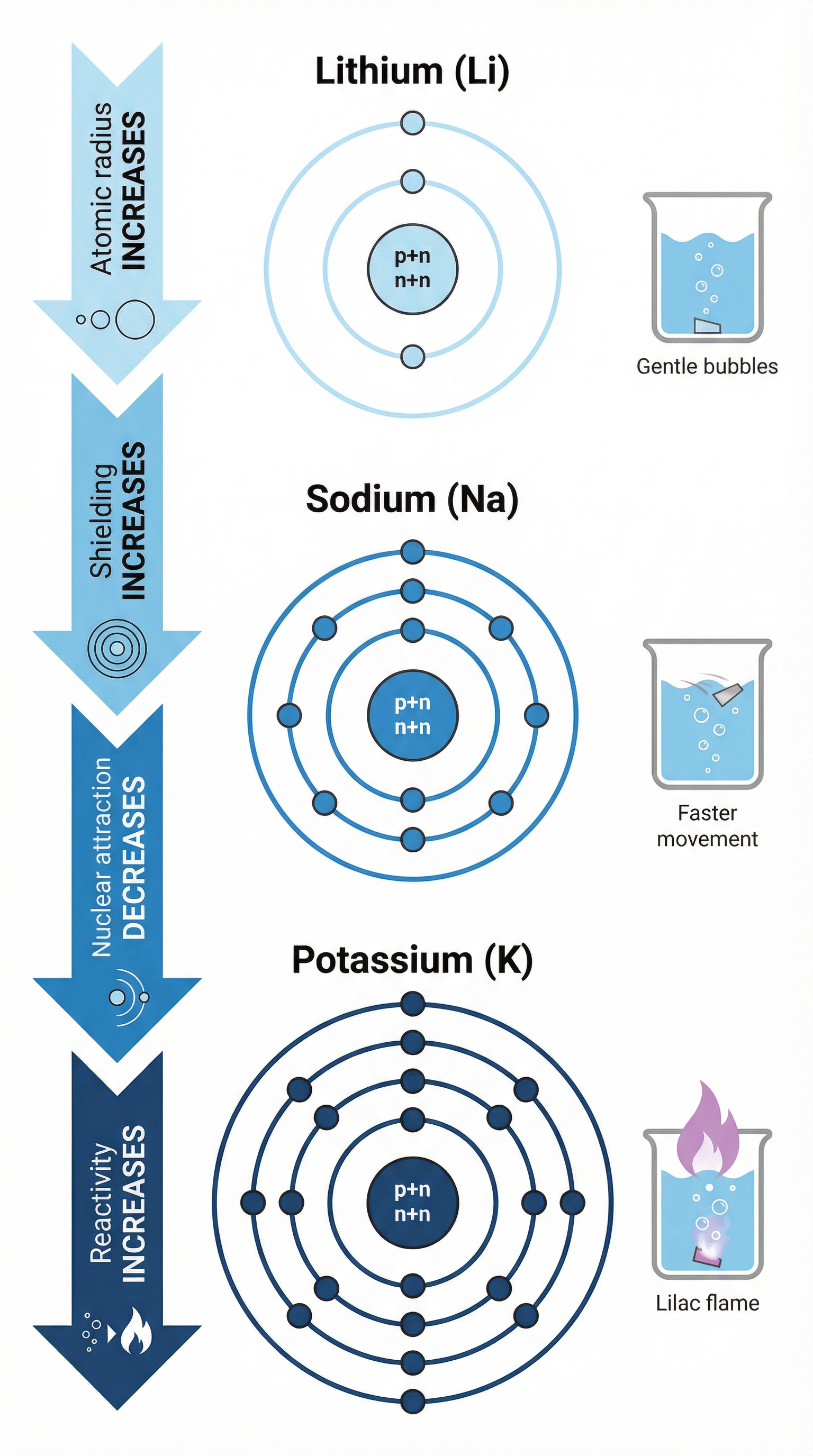
Group 1 elements are located in the first column of the periodic table and include Lithium (Li), Sodium (Na), and Potassium (K). They are called alkali metals because they form alkaline solutions when they react with water. These metals are characterized by having only one electron in their outer shell. This single electron is easily lost, making them very reactive metals. As you descend the group, the reactivity increases. This is a critical trend to remember and explain.
Why does reactivity increase down Group 1? (Higher Tier)
To explain this trend, you must refer to the atomic structure:
- Atomic Radius Increases: As you go down the group, each element has an additional electron shell, so the atom gets bigger.
- Shielding Increases: The inner shells of electrons shield the outer electron from the positive pull of the nucleus.
- Nuclear Attraction Decreases: The combination of a larger atomic radius and increased shielding means the attraction between the nucleus and the single outer electron weakens.
- Ease of Electron Loss: Consequently, less energy is needed to remove the outer electron. The easier it is to lose this electron, the more reactive the metal is.
Concept 2: Group 7 – The Halogens
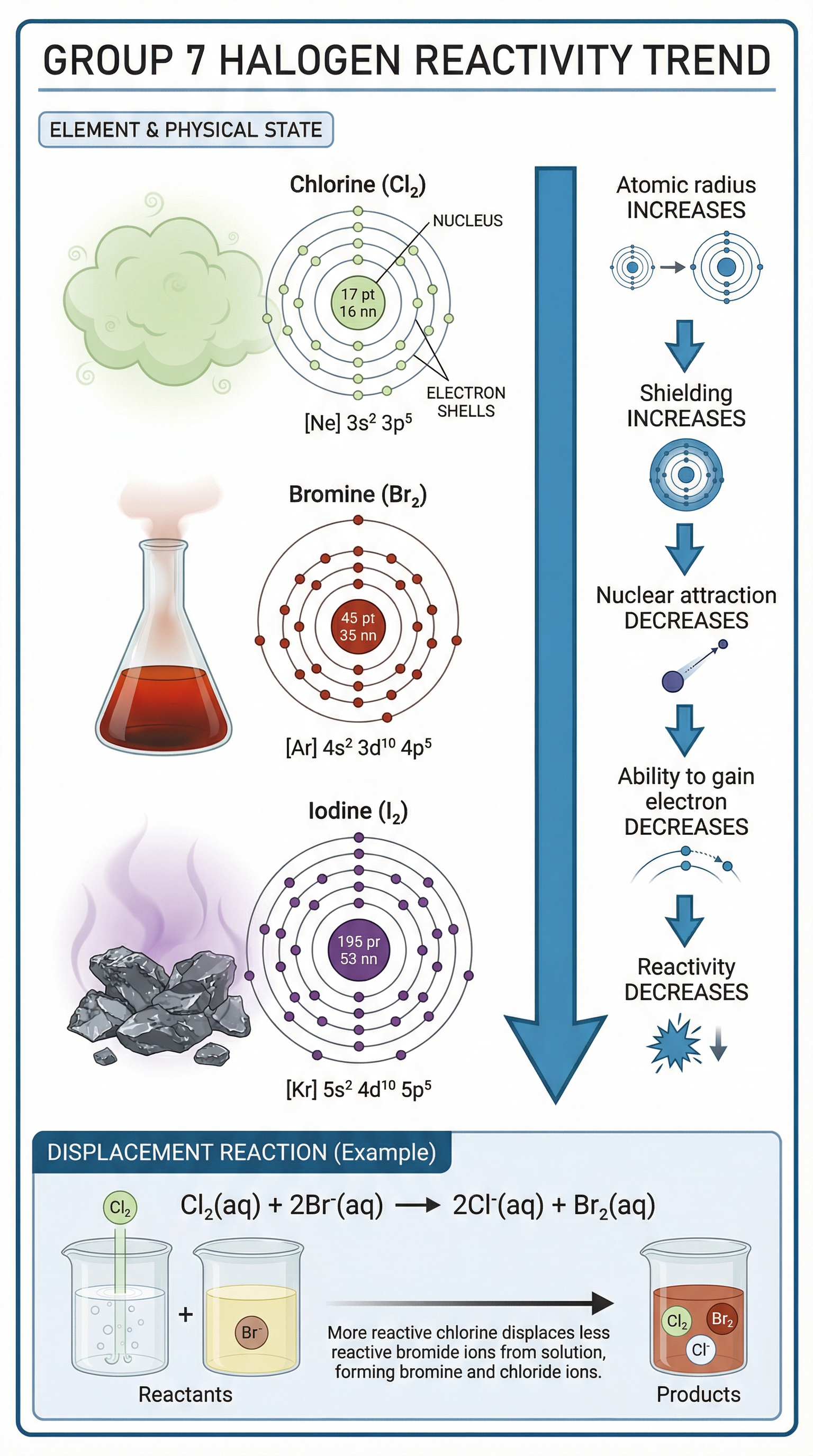
The halogens are in the second to last column of the periodic table and include Chlorine (Cl), Bromine (Br), and Iodine (I). They are reactive non-metals that exist as diatomic molecules (e.g., Cl₂, Br₂). Halogens have seven electrons in their outer shell, meaning they only need to gain one more electron to achieve a stable, full outer shell. This drive to gain an electron makes them reactive. In direct contrast to Group 1, as you descend Group 7, the reactivity decreases.
Why does reactivity decrease down Group 7? (Higher Tier)
The reasoning is similar to Group 1 but applied to gaining an electron:
- Atomic Radius Increases: More electron shells mean a larger atom.
- Shielding Increases: More inner shells shield the nucleus.
- Nuclear Attraction Decreases: The positive pull of the nucleus on an incoming electron is weaker.
- Difficulty in Gaining an Electron: It becomes harder for the atom to attract and capture an electron to complete its outer shell. Therefore, reactivity decreases.
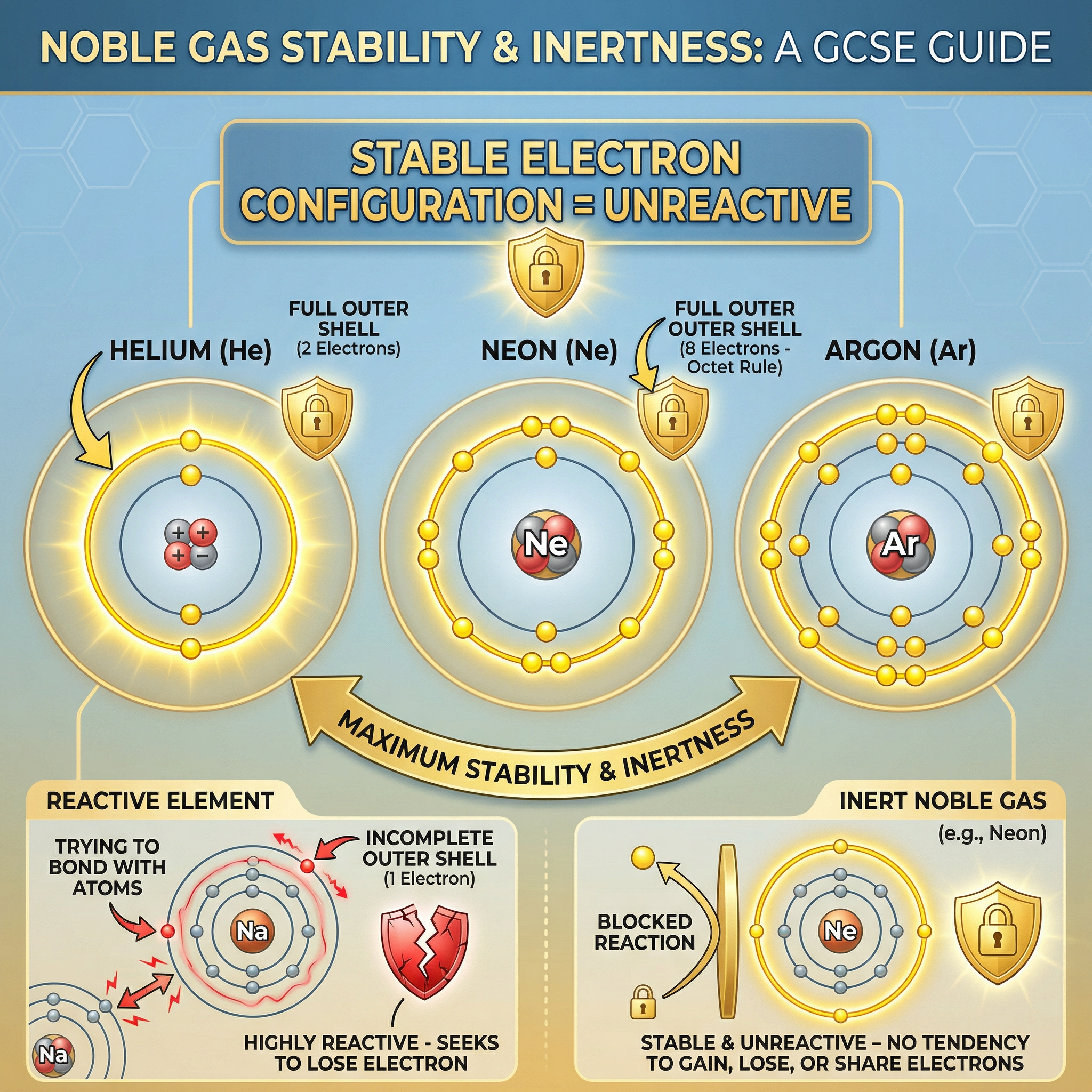
Concept 3: Group 0 – The Noble Gases
Group 0 elements, on the far right of the periodic table, include Helium (He), Neon (Ne), and Argon (Ar). They are exceptionally unreactive, or inert. This is because they have a full outer shell of electrons (2 for Helium, 8 for the others). This is a very stable electron configuration, so they have no tendency to lose, gain, or share electrons to form bonds. Their inertness makes them useful, for example, Argon is used in light bulbs to prevent the hot filament from reacting with oxygen.
Mathematical/Scientific Relationships
There are no mathematical formulas to memorise for this topic. However, you must be able to write and balance symbol equations for the reactions of alkali metals and the displacement reactions of halogens.
Alkali Metal + Water → Metal Hydroxide + Hydrogen
- 2Na(s) + 2H₂O(l) → 2NaOH(aq) + H₂(g)
Halogen Displacement Reaction
- Cl₂(aq) + 2KBr(aq) → 2KCl(aq) + Br₂(aq)
Practical Applications
- Alkali Metals: The vigorous reaction of alkali metals with water is a key demonstration. You must know the observations: fizzing (hydrogen gas produced), floating, and moving on the surface. For potassium, a lilac flame is also observed.
- Halogens: Halogen displacement reactions are a required practical topic. You would mix a halogen solution (e.g., chlorine water) with a halide salt solution (e.g., potassium bromide) and observe any colour change, which indicates a reaction has occurred. A more reactive halogen displaces a less reactive one.
- Noble Gases: Their inertness is their main application. Argon is used in welding to provide an inert atmosphere and prevent the hot metal from oxidizing. Neon is used in advertising signs because it glows when electricity is passed through it.
