Study Notes
Overview
The Particle Model is a cornerstone of GCSE Combined Science, providing the foundation for understanding the physical world. This topic explores how all matter is composed of tiny, moving particles and how their energy and arrangement determine whether a substance is a solid, liquid, or gas. For your Edexcel exam, you will be expected to use this model to explain phenomena like density, changes of state, and gas pressure. A strong grasp of this topic is essential as it links directly to energy, forces, and chemical reactions. Examiners frequently test this area with a mix of calculation questions (like density), data interpretation (like heating curves), and longer written explanations requiring you to apply the model to specific scenarios.
Key Concepts
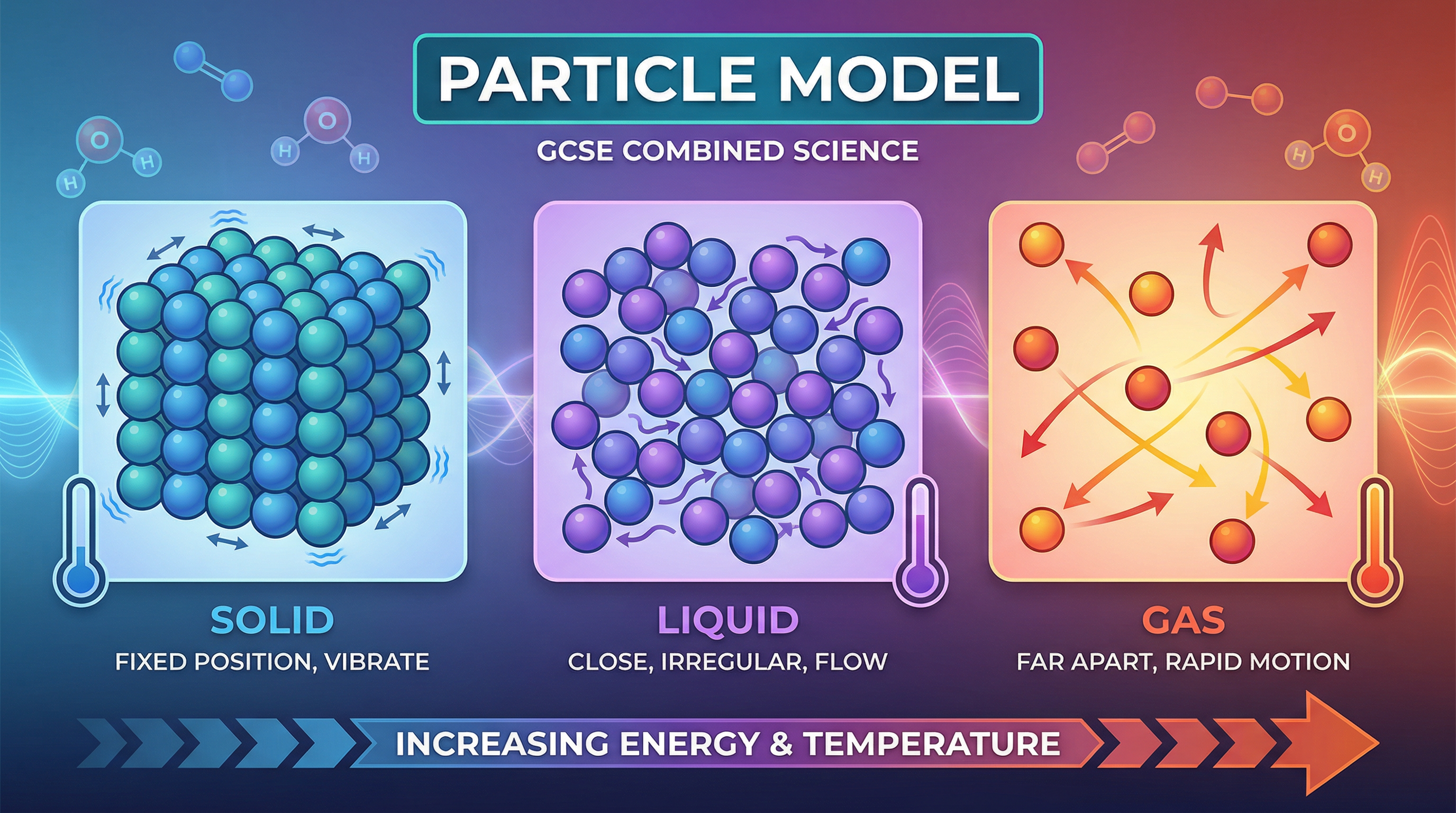
Concept 1: States of Matter
All substances are made of particles, but the arrangement and energy of these particles differ, leading to the three states of matter: solid, liquid, and gas. In a solid, particles are held in fixed positions within a regular lattice. They have strong forces of attraction between them, so they can only vibrate. This is why solids have a definite shape and cannot be compressed. In a liquid, particles are still in close contact but are arranged randomly and can move past one another. The forces between them are weaker than in solids, allowing liquids to flow and take the shape of their container. In a gas, particles are far apart and move randomly and rapidly. The forces between them are negligible. This is why gases fill any container and are easily compressed.
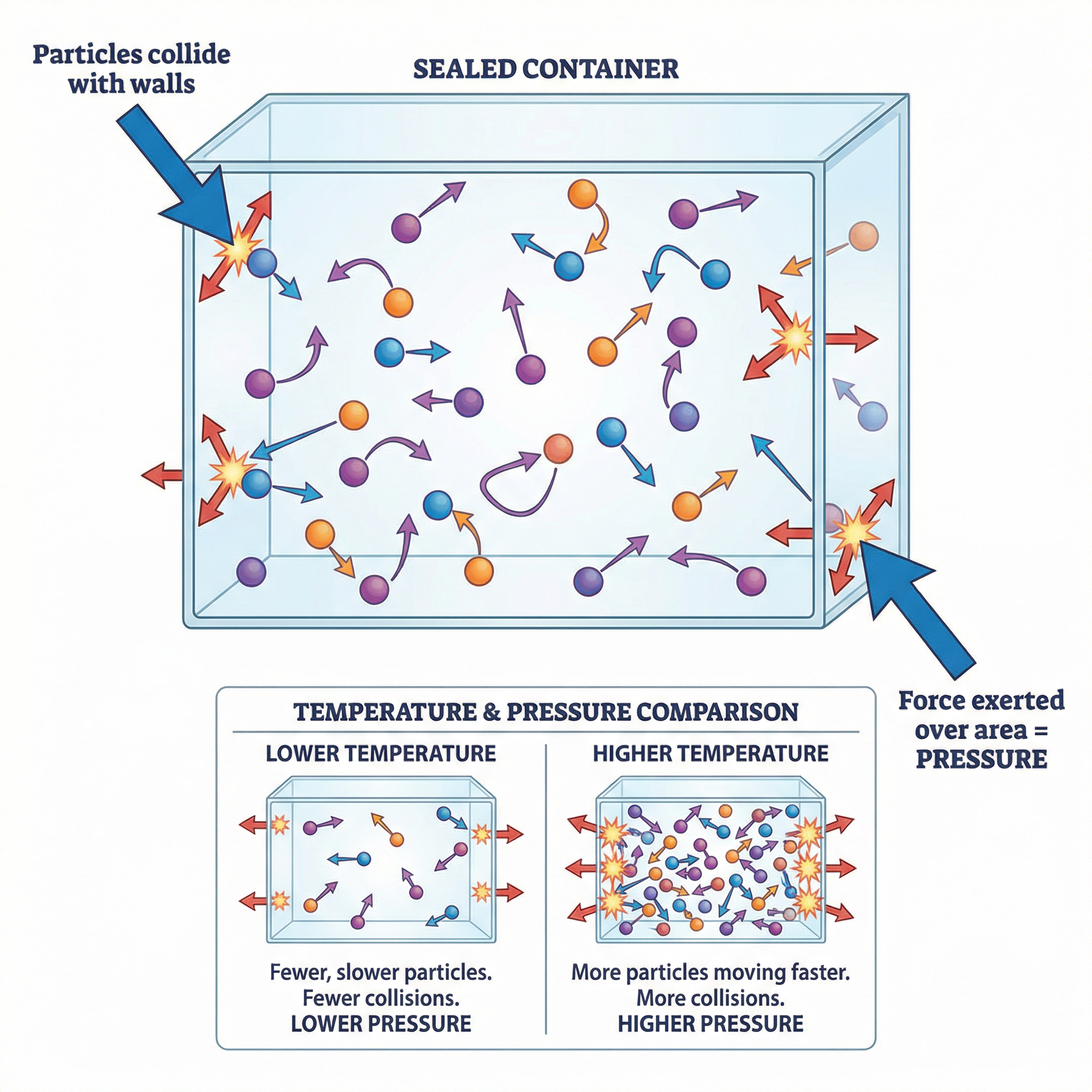
Concept 2: Density
Density is a measure of how much mass is packed into a certain volume. It's a fundamental property that helps us understand why some objects float and others sink. The key relationship to memorise is the formula:
Density (kg/m³) = Mass (kg) / Volume (m³) or ρ = m/VFor your exam, you must be confident in calculating density for both regular and irregular objects. This is a required practical skill.
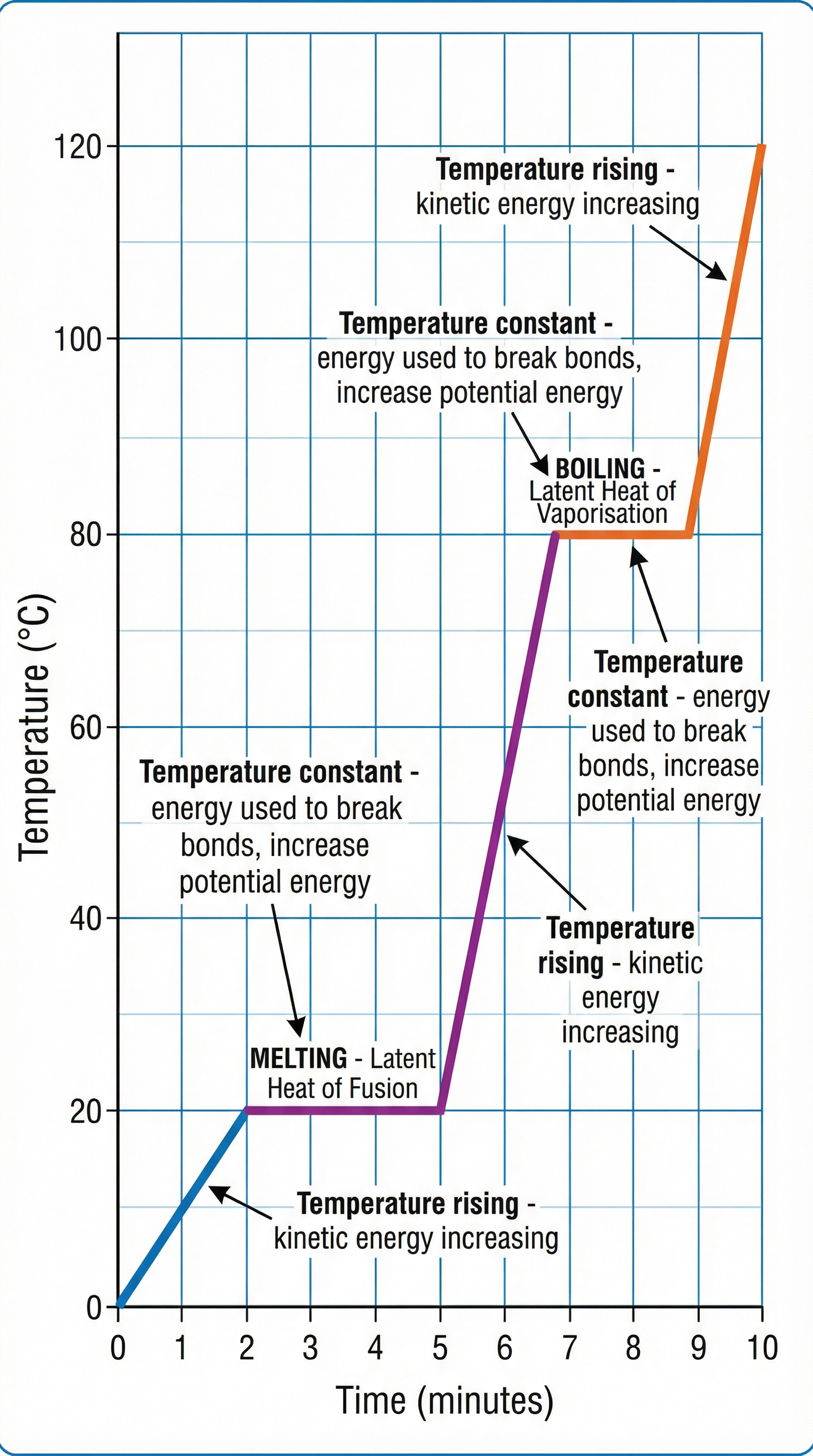
Concept 3: Changes of State & Latent Heat
When a substance is heated, its particles gain energy. This can either increase their kinetic energy (making them move faster and thus increasing the temperature) or increase their potential energy (breaking the bonds between them, causing a change of state). During a change of state, the temperature remains constant because the energy being supplied is used to overcome the forces of attraction between particles. This energy is called latent heat. The flat sections on a heating curve show this process in action. Specific Latent Heat of Fusion is the energy needed to change 1kg of a substance from solid to liquid at its melting point. Specific Latent Heat of Vaporisation is the energy needed to change 1kg of a substance from liquid to gas at its boiling point.
Mathematical/Scientific Relationships
-
Density Formula (Must memorise):
- ρ = m / V
- ρ (rho) = density (in kg/m³ or g/cm³)
- m = mass (in kg or g)
- V = volume (in m³ or cm³)
-
Specific Heat Capacity (Given on formula sheet):
- ΔE = m × c × Δθ
- ΔE = change in thermal energy (in Joules, J)
- m = mass (in kg)
- c = specific heat capacity (in J/kg°C)
- Δθ = change in temperature (in °C)
-
Specific Latent Heat (Given on formula sheet):
- E = m × L
- E = energy for a change of state (in Joules, J)
- m = mass (in kg)
- L = specific latent heat (in J/kg)
Practical Applications
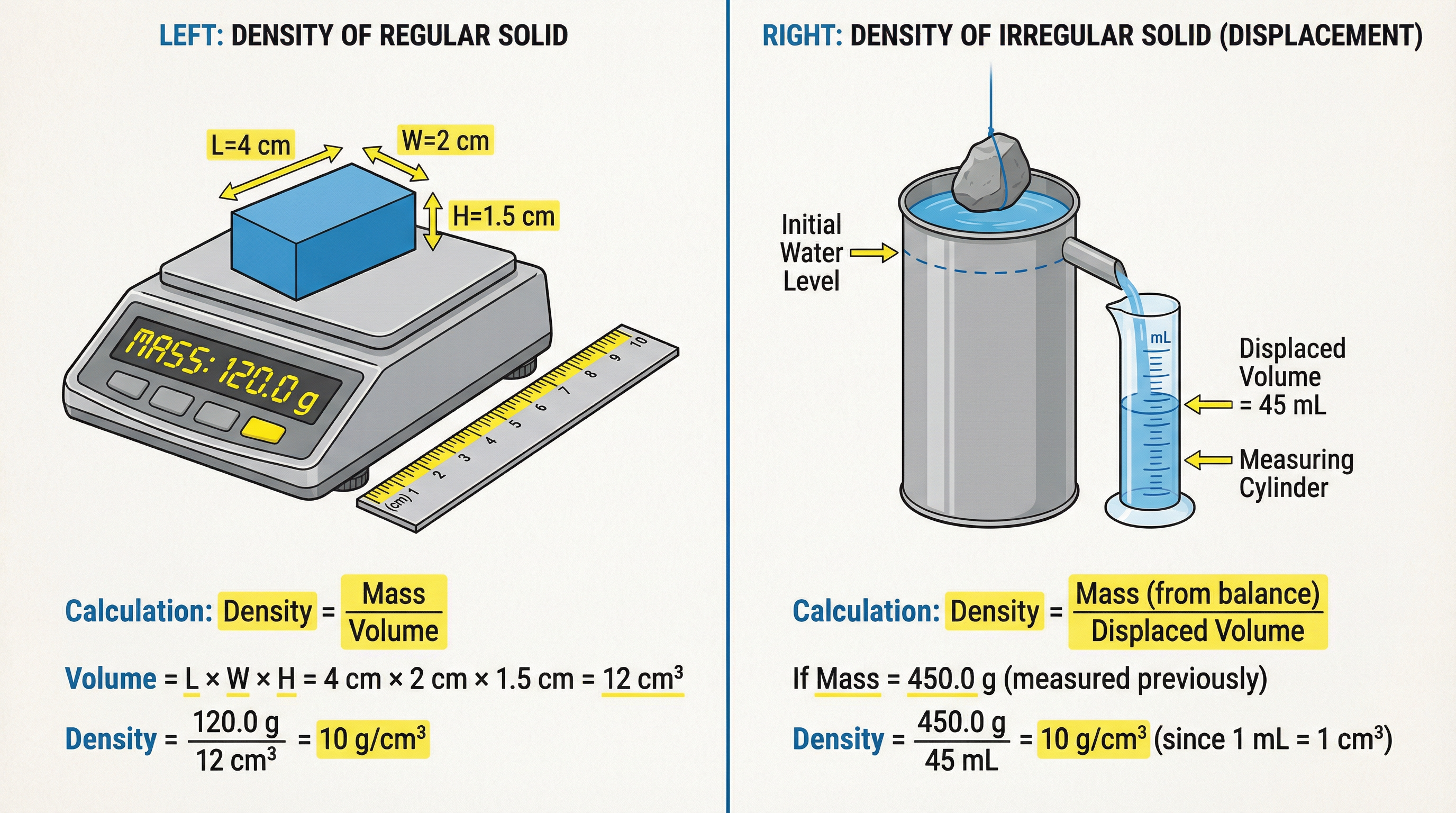
This topic is directly assessed through the Core Practical: Determining Density. You need to know the method for both regular and irregular solids.
Apparatus:
- Digital balance (for mass)
- Ruler (for regular solids)
- Eureka (displacement) can, measuring cylinder (for irregular solids)
- Water
Method for Irregular Solid:
- Measure the mass of the object using a digital balance.
- Fill the Eureka can with water until it just starts to flow from the spout.
- Place an empty measuring cylinder under the spout.
- Carefully lower the object into the Eureka can, ensuring it is fully submerged.
- Collect the displaced water in the measuring cylinder. The volume of this water is equal to the volume of the object.
- Calculate density using ρ = m/V.
Common Errors:
- Misreading the volume from the measuring cylinder (not reading from the bottom of the meniscus).
- Water splashing out of the can, leading to an inaccurate volume measurement.
- Forgetting to convert units, especially cm³ to m³ (divide by 1,000,000).
