Study Notes
Overview
Welcome to the core of practical chemistry: predicting and identifying reactions. This topic (OCR specification reference 2.4) is fundamental because it moves beyond just knowing chemical names and into the realm of understanding how chemicals behave. Examiners frequently test this area because it reveals a candidate's true grasp of chemical principles. You'll be expected to apply general reaction schemes to new situations, construct balanced equations, and interpret observations. Mastering this isn't just about memorising; it's about learning a set of rules that unlocks the ability to predict the future of a chemical reaction. This skill is not only crucial for your GCSE but forms the bedrock of A-Level chemistry, particularly in topics like redox, rates of reaction, and chemical analysis.
Key Concepts
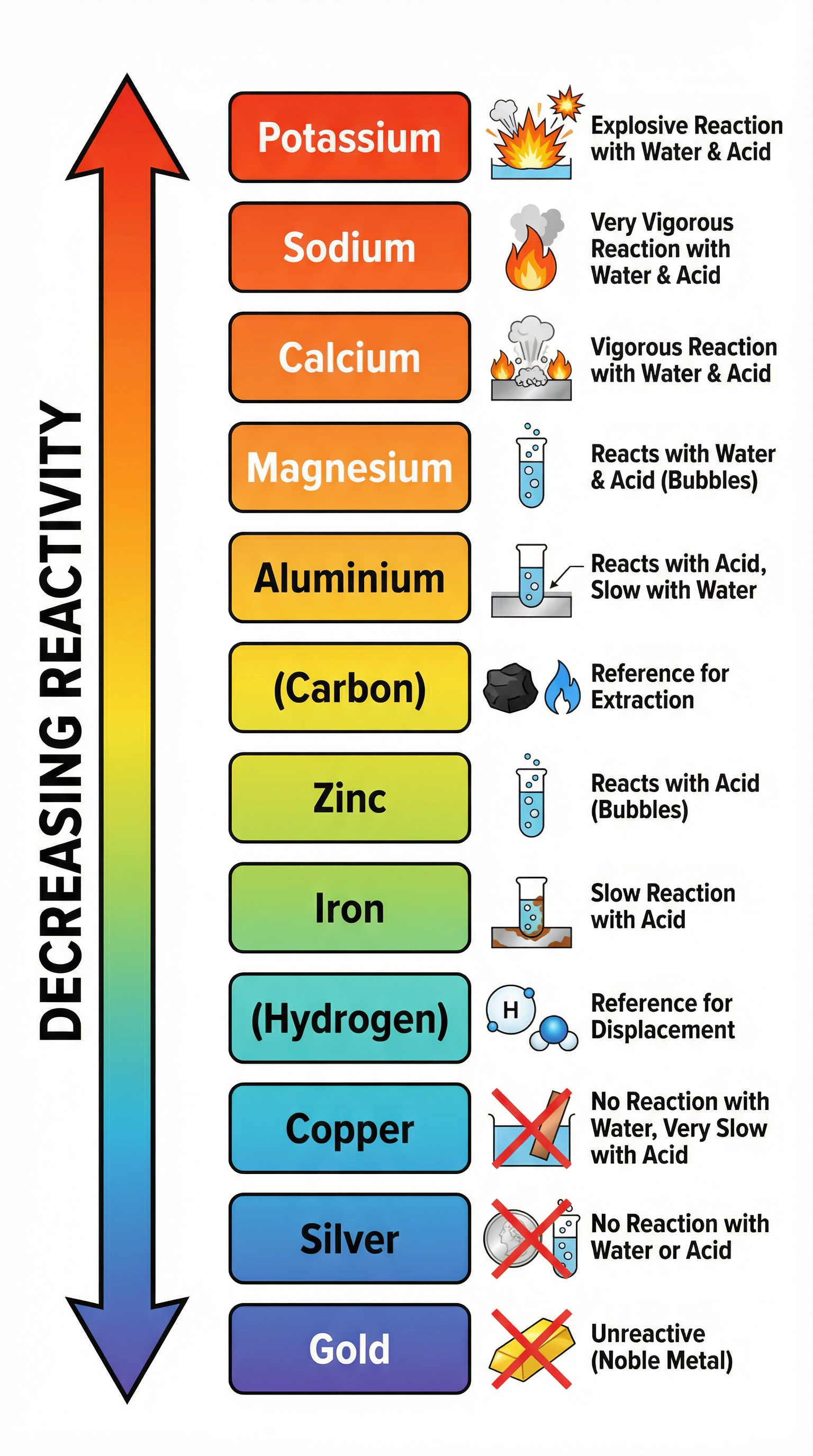
Concept 1: The Reactivity Series
The reactivity series is a league table for metals, ranking them from most to least reactive. This is one of the most important tools for predicting reactions. A more reactive metal can displace (push out) a less reactive metal from its compound. For example, magnesium is more reactive than copper, so if you add magnesium to copper sulfate solution, the magnesium will displace the copper, forming magnesium sulfate and solid copper. Examiners love asking about this.
Example: Mg(s) + CuSO4(aq) -> MgSO4(aq) + Cu(s)
Concept 2: The Four General Acid Reactions
Almost every acid reaction you'll encounter at GCSE falls into one of four patterns. Learn these, and you can handle almost any question. These are your chemical toolkit.
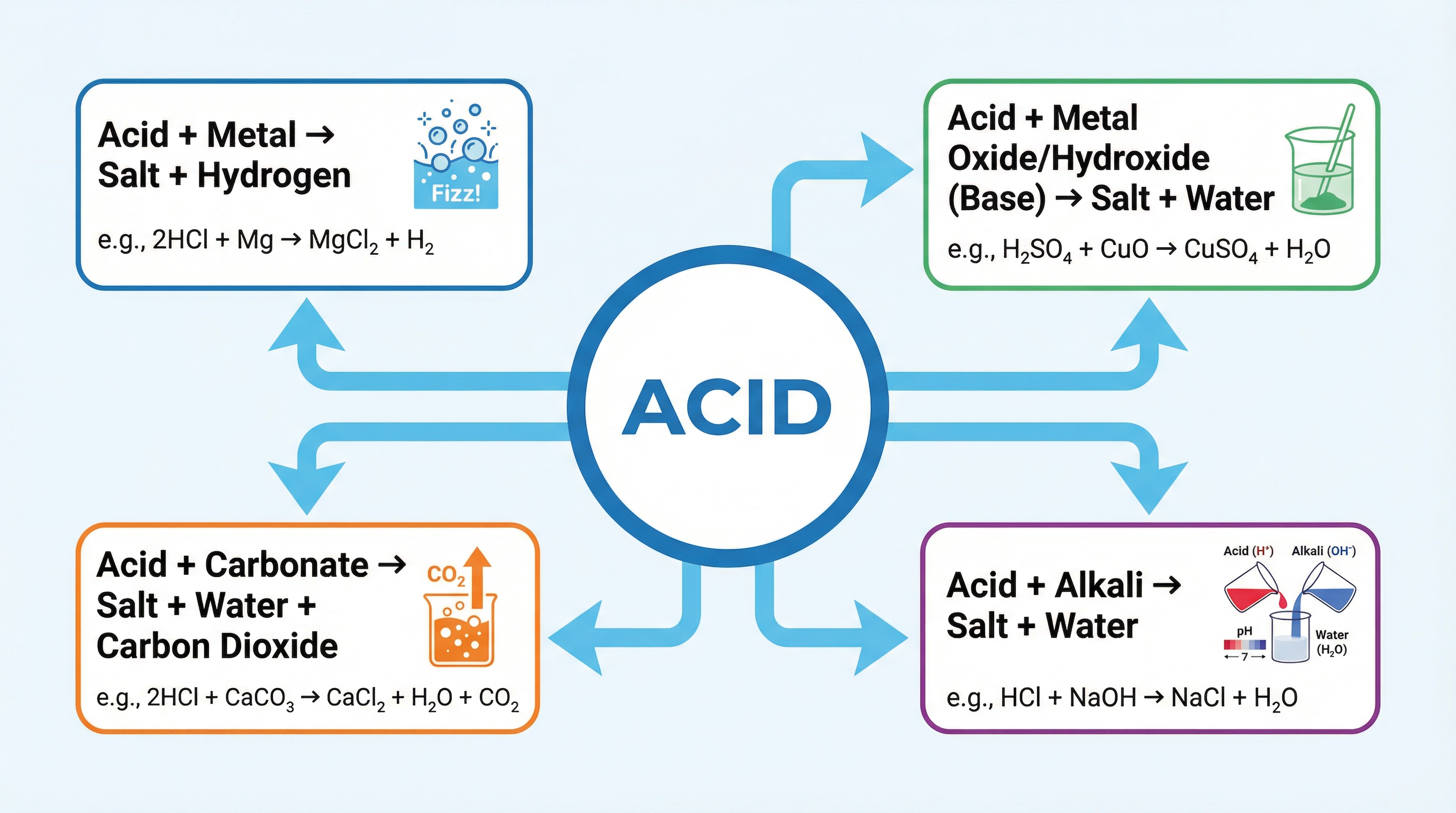
1. Acid + Metal -> Salt + HydrogenThis reaction only works for metals that are more reactive than hydrogen. The salt's name comes from the metal and the acid (e.g., Magnesium + Hydrochloric Acid -> Magnesium Chloride). The key observation is fizzing, which is the hydrogen gas being produced.
2. Acid + Base (Metal Oxide/Hydroxide) -> Salt + WaterThis is a neutralisation reaction. The base cancels out the acid. The only products are a salt and water. You'll often see the solid base 'disappearing' as it reacts and dissolves in the acid.
3. Acid + Carbonate -> Salt + Water + Carbon DioxideThis is a crucial one to remember as it has three products. The fizzing you see is carbon dioxide gas. The test for CO2 is that it turns limewater (calcium hydroxide solution) cloudy.
4. Acid + Alkali (Soluble Base) -> Salt + WaterThis is another neutralisation reaction, but since the alkali is already dissolved, you often don't see any change unless you use a pH indicator. This is the basis of titrations.
Concept 3: Balancing Equations
Balancing an equation ensures the law of conservation of mass is obeyed - no atoms are created or destroyed. The golden rule is: you can only change the large numbers in front of the formulas (coefficients), never the small numbers within them (subscripts). Changing a subscript changes the chemical itself!
Example: To balance H2 + O2 -> H2O, you need two hydrogens and two oxygens on both sides. The formula H2O is fixed. You can't write H2O2. Instead, you need two water molecules: H2 + O2 -> 2H2O. But now you have 4 hydrogens on the right, so you need 2 hydrogen molecules on the left: 2H2 + O2 -> 2H2O. Now it's balanced.
Concept 4: State Symbols
State symbols are required for full marks in equation questions. They are: (s) for solid, (l) for liquid, (g) for gas, and (aq) for aqueous (dissolved in water). Common mistakes include writing water as (aq) (it should be (l)) or salts formed in solution as (s) (they should be (aq)).
Mathematical/Scientific Relationships
- General Acid Equations (Must memorise):
- Acid + Metal -> Salt + H2(g)
- Acid + Base -> Salt + H2O(l)
- Acid + Carbonate -> Salt + H2O(l) + CO2(g)
- Acid + Alkali -> Salt + H2O(l)
- Ionic Equations (Higher Tier Only): These show only the species that change during the reaction. Spectator ions (which remain unchanged) are omitted. For neutralisation, the ionic equation is always:
H+(aq) + OH-(aq) -> H2O(l).
