Study Notes
Overview
Welcome to your deep dive into Bonding, Structure, and the Properties of Matter, topic 2.2 of the WJEC GCSE Combined Science specification. This topic is a cornerstone of chemistry, explaining how and why atoms join together to form the vast array of substances we see and interact with every day. A solid understanding here is not just about memorising definitions; it’s about linking the invisible world of atoms and electrons to the visible, tangible properties of materials. Examiners frequently test this through high-mark extended response questions (QERs), so mastering the connections between bonding, structure, and properties is essential for achieving a high grade. This guide will equip you with the precise language and conceptual understanding needed to confidently tackle any question the exam throws at you.
Key Concepts
Concept 1: Ionic Bonding
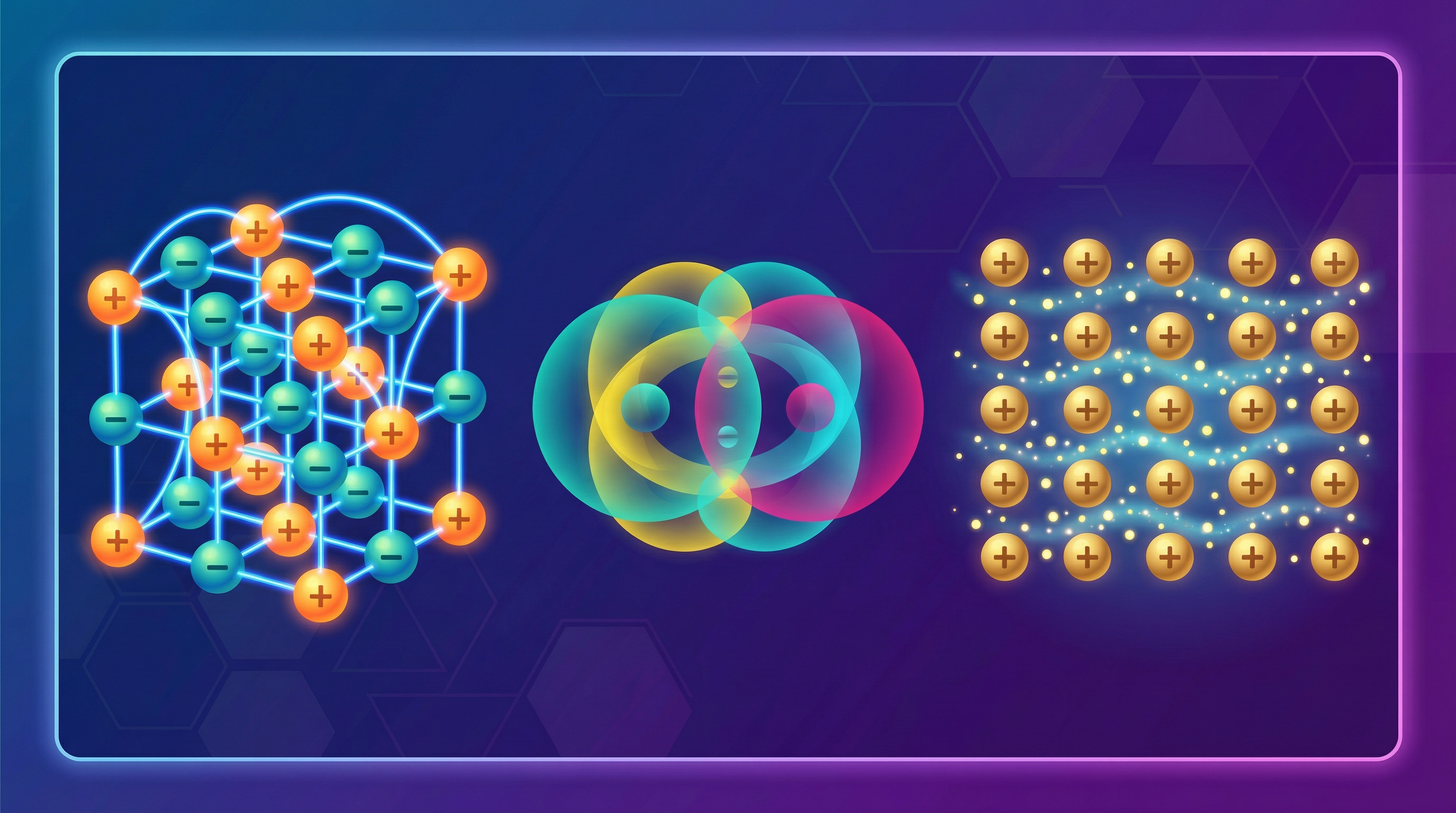
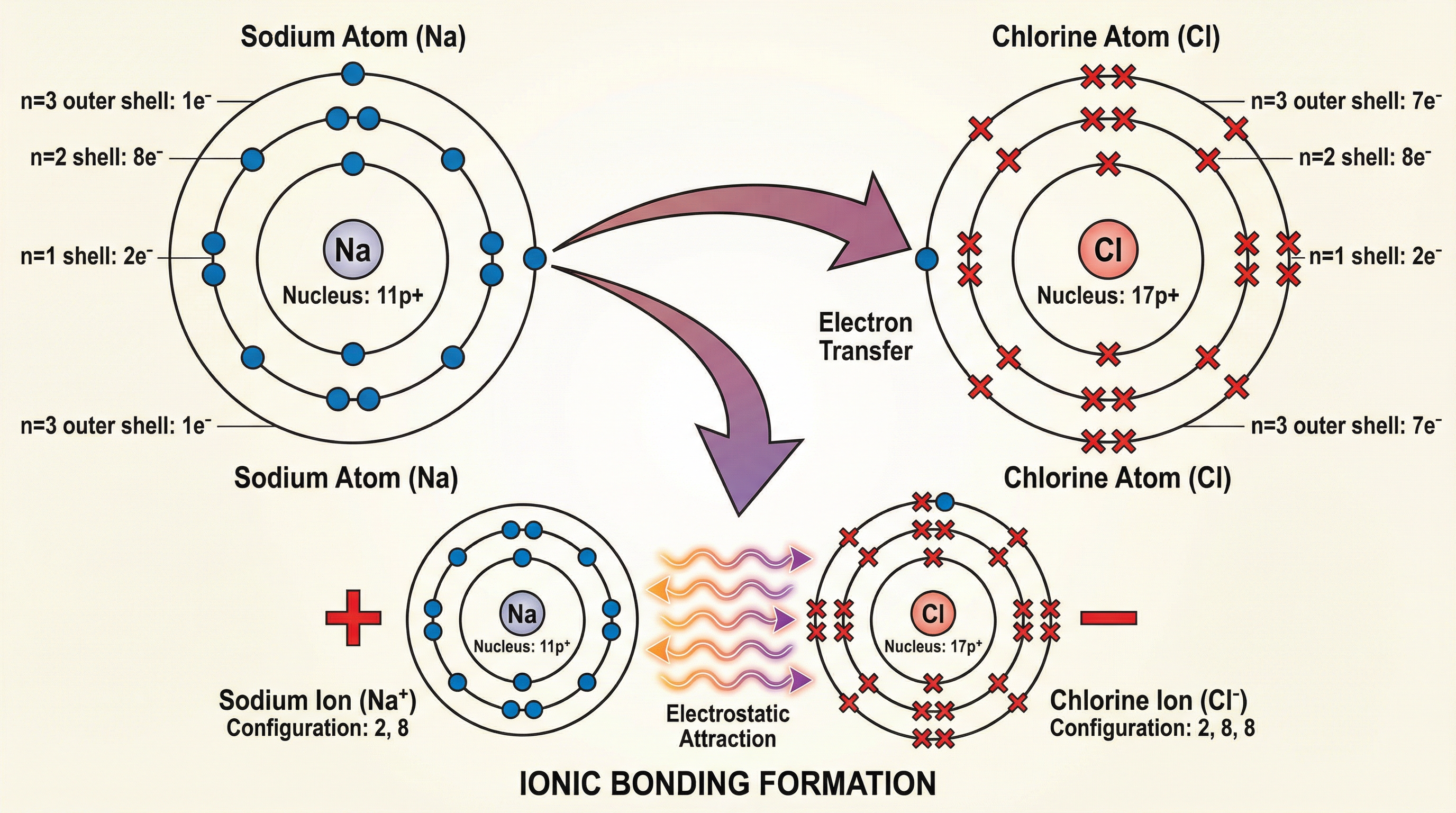
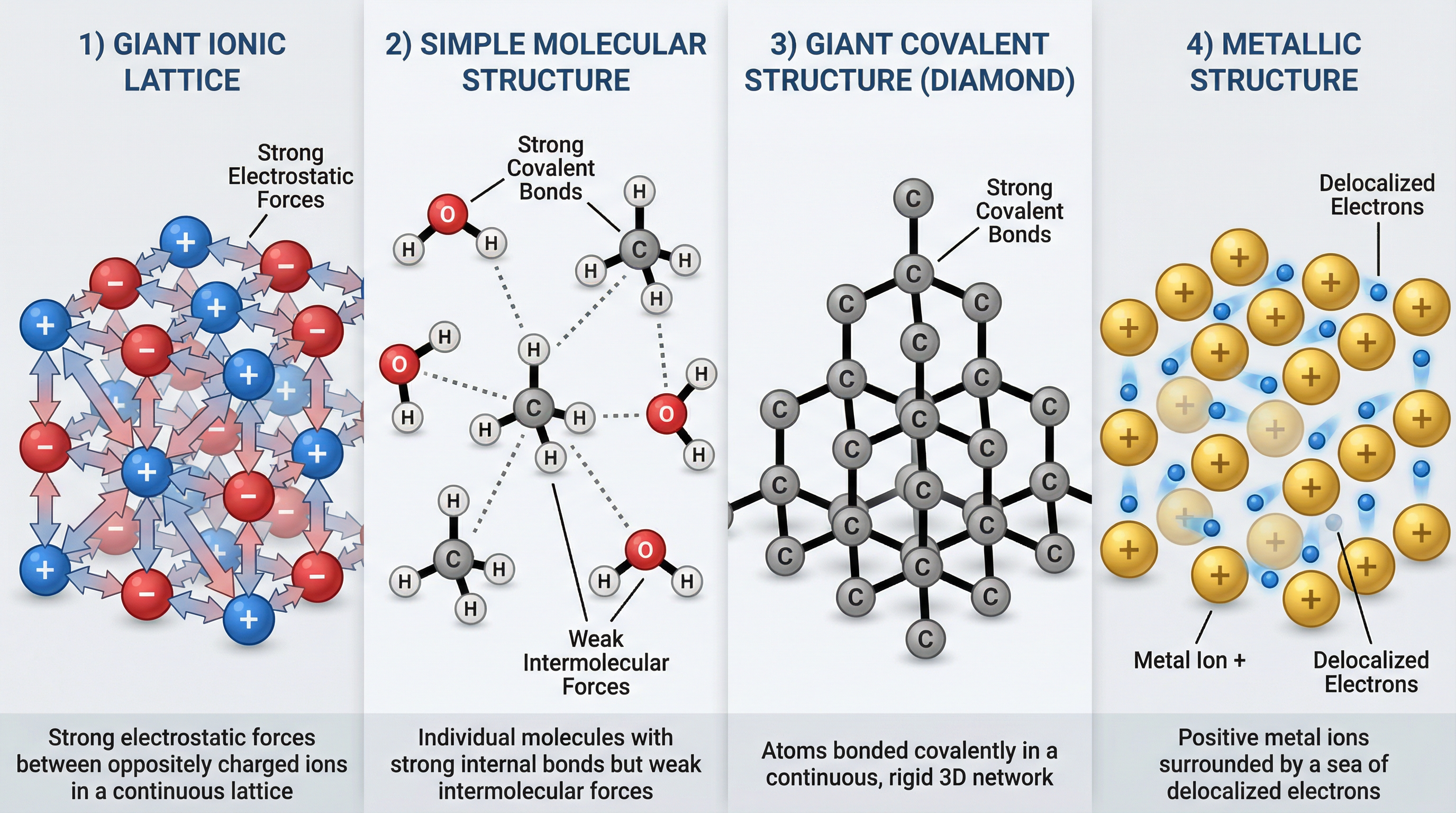
Ionic bonding occurs between a metal and a non-metal. It is driven by the desire of atoms to achieve a stable, full outer shell of electrons, just like the noble gases. Metal atoms have few electrons in their outer shell and lose them to form positive ions (cations). Non-metal atoms have nearly full outer shells and gain electrons to form negative ions (anions). The core of ionic bonding is the electrostatic attraction between these oppositely charged ions. It’s not just a simple pairing; these forces are strong and act in all directions, causing the ions to arrange themselves into a highly ordered, three-dimensional structure called a giant ionic lattice.
Example: Sodium (a Group 1 metal) reacts with Chlorine (a Group 7 non-metal). Sodium has one electron in its outer shell, which it transfers to Chlorine, which has seven. Sodium becomes a Na+ ion, and Chlorine becomes a Cl- ion. The strong electrostatic attraction between them forms Sodium Chloride (table salt).
Concept 2: Covalent Bonding
Covalent bonding happens between non-metal atoms. Instead of transferring electrons, the atoms share one or more pairs of electrons to achieve a full outer shell. A shared pair of electrons forms a strong covalent bond that holds the atoms together within a molecule. This type of bonding leads to two distinct types of structures that you must be able to differentiate.
- Simple Molecular Structures: These consist of a fixed number of atoms joined by strong covalent bonds (e.g., water, H₂O; methane, CH₄; carbon dioxide, CO₂). Crucially, while the covalent bonds within the molecules are very strong, the forces between the molecules are weak intermolecular forces. It is these weak forces that are overcome when the substance melts or boils, not the strong covalent bonds. This is a key point that many candidates get wrong.
- Giant Covalent Structures: In these structures, a vast number of atoms are all joined together by a network of strong covalent bonds. There are no separate molecules. This results in substances with extremely high melting and boiling points because a huge amount of energy is needed to break the countless strong covalent bonds. Examples include diamond and graphite (allotropes of carbon) and silicon dioxide.
Concept 3: Metallic Bonding
Metallic bonding is found, as the name suggests, in metals and alloys. It consists of a regular, repeating lattice of positive metal ions surrounded by a ‘sea’ of delocalized electrons. These delocalized electrons are the outer shell electrons from each metal atom, which are no longer associated with any single atom and are free to move throughout the entire structure. The metallic bond is the strong electrostatic attraction between the positive metal ions and the delocalized electrons. This unique structure is responsible for the characteristic properties of metals.
Mathematical/Scientific Relationships
There are no specific mathematical formulas to memorise for this topic. However, you must be proficient in drawing dot-and-cross diagrams to represent ionic and covalent bonding. Remember:
- Use different symbols (dots and crosses) for electrons from different atoms.
- For ionic bonding, show the electron transfer with an arrow and then draw the resulting ions with their charges and full outer shells in square brackets.
- For covalent bonding, show the shared pair(s) of electrons in the overlapping region of the outer shells.
Practical Applications
- Ionic Compounds: Used in everything from food seasoning (NaCl) to plaster of Paris (hydrated calcium sulfate). Their high melting points make them suitable for use as furnace linings (e.g., MgO).
- Simple Covalent Molecules: Many are essential for life (water, oxygen, carbon dioxide). Their low boiling points mean they are often gases or liquids at room temperature.
- Giant Covalent Structures: Diamond's hardness makes it ideal for cutting tools. Graphite's conductivity and softness make it perfect for electrodes and as a lubricant.
- Metals: Their conductivity makes them essential for electrical wiring (copper). Their strength and malleability make them vital for construction (iron/steel) and manufacturing (aluminium).
